(Cd) Cadmium NMR
Use our NMR service that provides Cd NMR and many other NMR techniques.
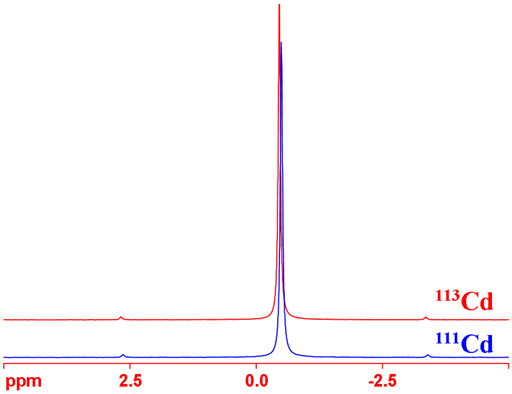
Cadmium has two NMR active spin ½ nuclei, 111Cd and 113Cd, that yield narrow signals (fig. 1) over a wide chemical shift range. 113Cd is very slightly the more sensitive nucleus and is therefore usually the preferred nucleus. Cadmium NMR is used for the study organocadmium compounds, cadmium complexes, inorganic cadmium and cadmium binding to proteins and other molecules of biological interest.
Fig. 1. Comparison of the (proton decoupled) NMR spectra of the cadmium isotopes 111Cd and 113Cd in neat dimethyl cadmium
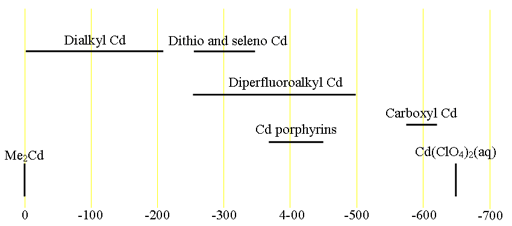
Each type of cadmium compound has its characteristic chemical shift range (fig. 2).
Fig. 2. Chemical shift ranges for cadmium NMR
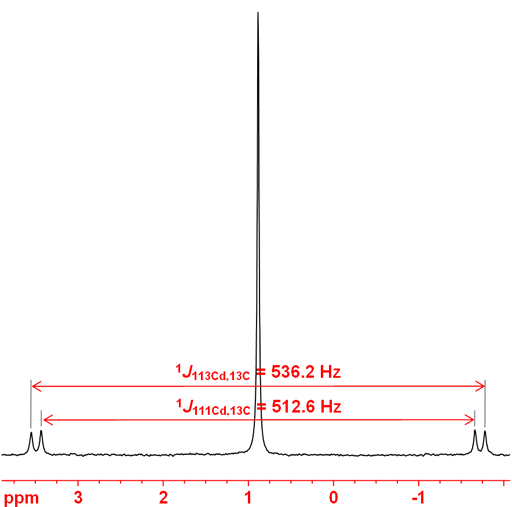
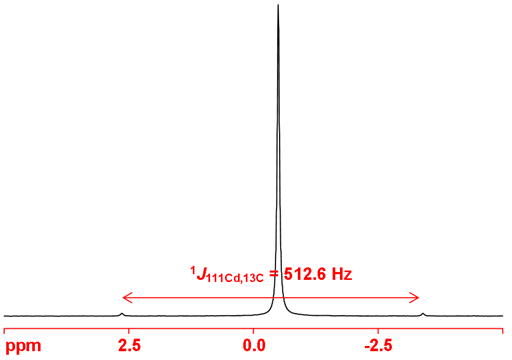
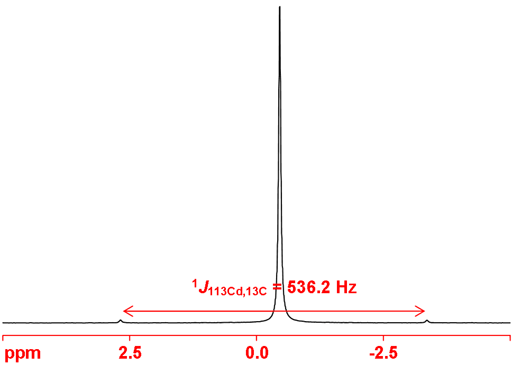
Both cadmium nuclei couple to other nuclei. 1H, 13C, 31P and 77Se couplings have been reported. On the coupling nucleus two set of couplings are observed as satellites as in the 13C spectrum in fig. 3. One bond couplings to 13C are typically around 500 Hz with the 111Cd coupling slightly smaller than the 113Cd coupling.
Fig. 3. 13C-NMR spectrum of Me2Cd showing coupling to both cadmium nuclei
Two and three bond couplings to 1H are typically in the region of 50 Hz. The difference between the 111Cd and 113Cd coupling constants with 1H is small so appear merged in the spectrum in fig. 4.
Fig. 4. 1H-NMR spectrum of Me2Cd showing apparently a single coupling to both cadmium nuclei
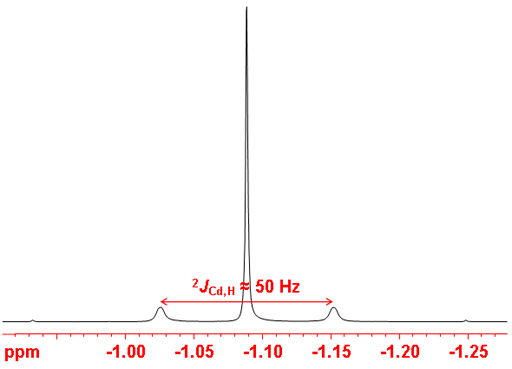
Resolution enhancment sepearates the two couplings (fig. 5).
Fig. 5. 1H-NMR spectrum of Me2Cd showing separate couplings to each cadmium nucleus

111Cadmium NMR
(111Cd) 111Cadmium is slightly less sensitive than 113Cd so is not usually the preferred nucleus of cadmium. It is a spin ½ nucleus and yields sharp signals (fig. 6).
Fig. 6. 111Cd-NMR spectrum of neat dimethyl cadmium showing coupling to 13C as satellite signals
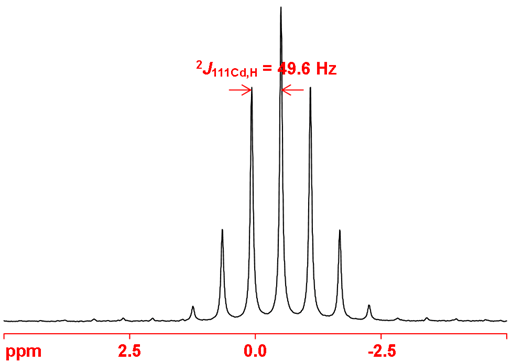
Cadmium often shows couplings to other nuclei, 1H, 13C, 19F, etc. Cadmium couplings to protons can be removed by decoupling as in fig. 6. The coupled spectrum of dimethyl cadmium (fig. 7) shows a two bond coupling to 1H, (2J111Cd,H) of 49.6 Hz.
Fig. 7. 111Cd-NMR spectrum of neat dimethyl cadmium showing coupling to 1H
Properties of 111Cd
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 12.80% |
| Chemical shift range | 650 ppm, from -650 to 0 |
| Frequency ratio (Ξ) | 21.215480% |
| Reference compound | Me2Cd |
| Linewidth of reference | 2.7 Hz |
| T1 of reference | 0.65 s |
| Receptivity rel. to 1H at natural abundance | 1.24 × 10-3 |
| Receptivity rel. to 1H when enriched | 6.69 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 7.27 |
| Receptivity rel. to 13C when enriched | 59.8 |
113Cadmium NMR
(113Cd) 113Cadmium is slightly more sensitive than 111Cd so is usually the preferred nucleus of cadmium. It is a spin ½ nucleus and yields sharp signals (fig. 8).
Fig. 8. 113Cd-NMR spectrum of neat dimethyl cadmium showing coupling to 13C as satellite signals
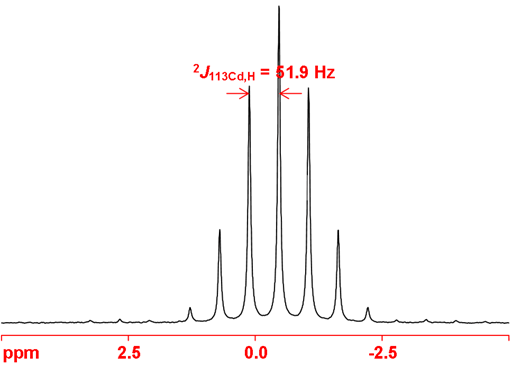
Cadmium often shows couplings to other nuclei, 1H, 13C, 19F, etc. Cadmium couplings to protons can be removed by decoupling as in fig. 8. The coupled spectrum of dimethyl cadmium (fig. 9) shows a two bond coupling to 1H, (2J113Cd,H) of 51.9 Hz.
Fig. 9. 113Cd-NMR spectrum of neat dimethyl cadmium showing coupling to 1H
Properties of 113Cd
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 12.22% |
| Chemical shift range | 650 ppm, from -650 to 0 |
| Frequency ratio (Ξ) | 22.193175% |
| Reference compound | Me2Cd |
| Linewidth of reference | 2.5 Hz |
| T1 of reference | 0.65 s |
| Receptivity rel. to 1H at natural abundance | 1.35 × 10-3 |
| Receptivity rel. to 1H when enriched | 0.0125 |
| Receptivity rel. to 13C at natural abundance | 7.94 |
| Receptivity rel. to 13C when enriched | 65.0 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, cadmium is a toxic carcinogen: wear protective gloves. Dimethyl cadmium is volatile: wear protective clothing and work in a hood. Latex gloves do not provide protection.
References
- G. E. Maciel and M. Borzo, "High resolution cadmium-113 nuclear magnetic resonance by pulse Fourier transform", J. Chem. Soc., Chem. Comm., 394 (1973).
- C. J. Turner and R. F. M. White, "Heteronuclear magnetic double-resonance studies of cadmium-113 shielding in dialkyl cadmium compounds", J. Magn. Res., 26, 1-5 (1977).
- I. M. Armitage and Y. Boulanger, "Cadmium-113 NMR", NMR Newly Accessible Nucl., 2, 337-365 (1983).
- J. L. Sudmeier, J. L. Evelhoch, S. J. Bell, M. C. Storm and M. F. Dunn, "Cadmium-113 NMR studies of insulin and calmodulin", Dev. Biochem., 14, 235-237 (1980).
- G. K. Carson and P. A. W. Dean, "A selenium-77 and cadmium-113 NMR spectroscopic study of phenylselenolate complexes of cadmium", Inorg. Chim. Acta, 66, 37-39 (1982).
- P. F. Rodesiler and E. L. Amma, "The solid and solution cadmium-113 NMR spectra of six and seven coordinate cadmium dications: relevance to calcium-substitution proteins", J. Chem. Soc., Chem. Comm., 182-184, (1982).
- Y. Boulanger, I. M. Armitage, K. A. Miklossy and D. R. Winge, "Cadmium-113 NMR study of a metallothionein fragment. Evidence for a two-domain structure", J. Biol. Chem., 257, 13717-13719 (1982).
- Structure elucidation of the metal-binding sites in metallothionein by cadmium -113 NMR. Armitage, Ian M.; Otvos, James D.; Briggs, Richard W.; Boulanger, Yvan. Dep. Mol. Biophys. Biochem., Yale Univ., New Haven, CT, USA. Federation Proceedings (1982), 41(13), 2974-80.
- P. Gettins and J. E. Coleman, "Cadmium-113 nuclear magnetic resonance of cadmium (II) alkaline phosphatases", J. Biol. Chem., 258, 397-407 (1983).
- J. K. Nicholson, P. J. Sadler, K. Cain, D. E. Holt, M. Webb and G. E. Hawkes, "88 MHz cadmium-113 NMR studies of native rat liver metallothioneins", Biochem. J., 211, 251-255 (1983).
- Cadmium-113 NMR. Armitage, Ian M.; Boulanger, Yvan. Dep. Mol. Biophys. Biochem., Yale Univ., New Haven, CT, USA. Editor(s): Laszlo, Pierre. NMR Newly Accessible Nucl., 2, 337-65 (1983).
- I. G. Dance, "Applications of cadmium NMR to polycadmium complexes. II. [Cdx(SPh)y]2x-y species in solution in relation to metallothionein aggregates", Inorg. Chim. Acta, 108, 227-230 (1985).
- I. G. Dance, "Applications of cadmium NMR to polycadmium complexes. III. Effects of zinc/cadmium and selenium/sulfur substitution in the tetraadamantanoid cage [S4Cd10(SPh)16]4- in relation to heterometal metallothioneins", Aust. J. Chem., 38, 1745-1755 (1985).
- I. G. Dance, R. G. Garbutt and D. C. Craig, "Applications of cadmium NMR to polycadmium complexes. IV. Reactions of [Cd10(SCH2CH2OH)16]4+ with chloride ion, and the crystal and molecular structure of hexadeca(2-hydroxyethanethiolato)tetrachlorodecacadmium [Cd10(SCH2CH2OH)16Cl4]", Aust. J. Chem., 39, 1339-1463 (1986).
- M. A. Kennedy and P. D. Ellis, "Cadmium-113 nuclear magnetic resonance spectroscopy of Cd2+-substituted heme and myoglobin", J. Am. Chem. Soc., 111, 3195-3203 (1989).
- J. E. Coleman, "Cadmium-113 nuclear magnetic resonance applied to metalloproteins", Methods in Enzymology, 227 (Metallobiochemistry, Pt. D) 16-43 (1993).
- F. He, X. Mao, "Study of the coordination sites of histidine to cadmium (II)", Bopuxue Zazhi, 13, 25-34 (1996).