19Flourine NMR
19Fluorine is a sensitive nucleus which yields sharp signals and has a wide chemical shift range.
A typical analysis of a 19F NMR spectrum may proceed similarly to that of Proton (1H). Our NMR service provides 19F NMR along with many other NMR techniques. The number of fluorines of each type in the spectrum of a pure sample can be obtained directly from the integrals of each multiplet provided that the multiplets are well separated which is very likely to the large chemical shift range. A routine NMR spectrum yields integrals with an accuracy of ±10%. Accuracies of ±1% can be achieved by increasing the relaxation delay to five times the longitudinal relaxation times (T1) of the signals of interest.
The multiplet structure yields information about the fluorine's neighborhood. Spin-spin couplings are transmitted through chemical bonds and yield information about the immediate molecular environment. The coupling constants are generally larger than those for 1H. Most fluorinated compounds contain hydrogen that couples with the fluorine in addition to homonuclear couplings. The multiplets may be split by other nuclei such as 31Phosphorus. If such heteronuclear couplings are undesirable they may be decoupled. The best pulse sequence in such a case is that for regular decoupling or for quantitative results, inverse gated decoupling.
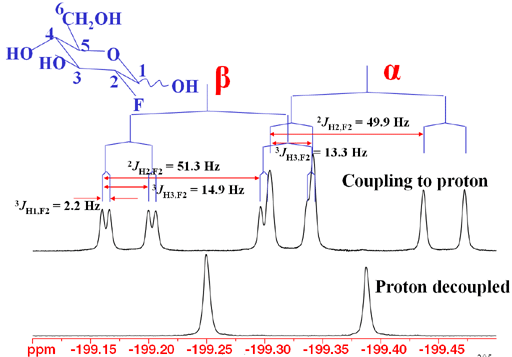
In the case of 2-fluoro-2-deoxyglucose, there are two anomers (a type of isomer specific to sugars) that yield two peaks in the decoupled fluorine spectrum and two overlapping multiplets in the coupled spectrum (Fig. 1).
Fig. 1. 19F Spectrum of 2-fluoro-2-deoxyglucose
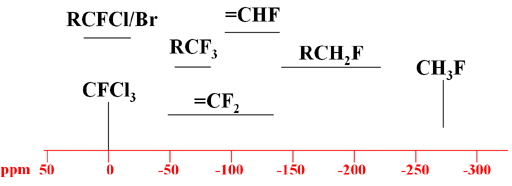
Each type of signal has a characteristic chemical shift range (Fig. 2).
Fig. 2. Typical chemical shift ranges for 19F
Properties of 19F
| Property | Value |
|---|---|
| Spin | ½ |
| Natural abundance | 100% |
| Chemical shift range | 700 ppm, from -300 to 400 |
| Frequency ratio (Ξ) | 94.094011% |
| Reference compound | CFCl3 = 0 ppm |
| Linewidth of reference | |
| T1 of reference | |
| Receptivity rel. to 1H at natural abundance | 0.83 |
| Receptivity rel. to 1H when enriched | 0.83 |
| Receptivity rel. to 13C at natural abundance | 4716 |
| Receptivity rel. to 13C when enriched | 4716 |
Safety of 19F-NMR
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, CFCl3 is toxic: wear protective gloves and work in a hood. Fluorides are toxic: wear protective gloves.