NMR Thermometer
Version 4.0
Use our NMR service.
Result
Please enter valid chemical shifts in order to see the temperature
The temperature of a sample in the probe of an NMR spectrometer can be determined from the separation between two peaks in a 1H NMR spectrum of a standard 'NMR thermometer' sample. Measure the 1H-NMR spectrum of the NMR thermometer standard of your choice under the same conditions as your experimental sample then use this app to determine the temperature. Deuterated samples should be locked and acquired as normal. Samples without deuterium enrichment (methanol and glycol) cannot be locked and should be tuned away from the resonance frequency in order to reduce saturation.
Select the NMR thermometer standard from the list:
- Deuterium oxide/DSS
- Deuterium oxide/TMS
- Glycol
- Glycol (80 wt%) in DMSO-d6
- Methanol
- Methanol-d4
- 2H of methanol-d4
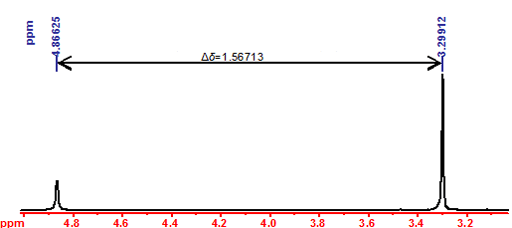
Choose if you want to enter two values for the individual chemical shifts or a single value for the separation between the peaks (Fig. 1).
Fig. 1. 1H NMR spectrum of methanol. The temperature is determined from the separation between the two peaks.
Enter the value(s) is the box(es) provided.
The temperature appears whenever valid number(s) are entered.
It is good practice to record the peak separation with the temperature so that the temperature can be recalculated if a better calibration becomes available.
If the parameters are in range the temperature will be provided in Kelvin, Celsius and Fahrenheit with accuracy constraints. If they are out of range, the temperature will be preceded by a warning that it is inaccurate. If the separation is wildly out of range or non-numeric parameters are inserted an appropriate warning is given instead of the temperature.
Preparation of standard samples
All deuterated solvents should be between 99.8 and 99.99% atom D. Non-aqueous solvents should contain less than 0.1% water. The apparent temperature measured with methanol and methanol-d4 increases by 0.7 °C per % water added and for glycol it decreases by 0.4 °C per % water added.
It is best to prepare the samples in sealed tubes for stability. TMS and DSS are best sealed under an inert atmosphere for long-term storage.
- Deuterium oxide/DSS Use between 0.01 and 0.1% DSS by mass.
- Deuterium oxide/TMS TMS is sparingly soluble in water. Prepare a saturated solution. A small layer of TMS can be allowed to remain above the D2O.
- Glycol A small amount of DMSO-d6 may be added to permit field-frequency locking. It is possible to remove water by pulling a vacuum to less than 0.1 mbar at room temperature prior to adding a partial pressure of dry gas and sealing the tube. Alternatively use exactly 20% by weight of DMSO-d6 and select the Glycol (80 wt%) in DMSO-d6 standard.
- Methanol Use dry methanol.
- Methanol-d4 A fresh ampoule of methanol-d4 is usually sufficiently pure.
Sources for calibration
Deuterium oxide/DSS: R. E. Hoffman "Standardization of chemical shifts of TMS and solvent signals in NMR solvents" Magn. Reson. Chem., 44, 606-616 (2006). This has been adjusted using the new methanol-d4 calibration (Karschin, et. al). T/°C = 398.1144 + 72.42468Δδ - 61.3641(Δδ)2 + 6.1147(Δδ)3
Deuterium oxide/TMS: R. E. Hoffman and E. D. Becker "Temperature dependence of the 1H chemical shift of temtramethylsilane in chloroform, methanol and dimethylsulfoxide" J. Magn. Reson., 176, 87-98 (2005). This has been adjusted using the new methanol-d4 calibration (Karschin, et. al). T/°C = 162.4065 + 241.5812Δδ - 99.6652(Δδ)2 + 9.0202(Δδ)3
Glycol: R. E. Hoffman and E. D. Becker R. E. Hoffman and E. D. Becker "Temperature dependence of the 1H chemical shift of temtramethylsilane in chloroform, methanol and dimethylsulfoxide" J. Magn. Reson., 176, 87-98 (2005). This calibration is a compramise between that published by C. Ammann, P. Meier and A. E. Merbach "A simple multinuclear NMR thermometer" J. Magn. Reson., 46, 319-321 (1982) and M. L. Kaplan, F. A. Bovey and H. N. Cheng "Simplified method of calibrating thermometric nuclear magnetic resonance standards" Anal. Chem., 47, 1703-1705 (1975). At the lower end of the range this has been adjusted using the new methanol-d4 calibration (Karschin, et. al). T/°C = 192.95 - 106.86Δδ + 15.316(Δδ)2 - 15.1926(Δδ)3 + 4.5385(Δδ)4. For 80% glycol in DMSO-d6: T/°C = 185.82 - 108.17Δδ + 13.306(Δδ)2 - 15.1926(Δδ)3 + 4.5385(Δδ)4
Methanol: C. Ammann, P. Meier and A. E. Merbach "A simple multinuclear NMR thermometer" J. Magn. Reson., 46, 319-321 (1982) This has been adjusted using the new methanol-d4 calibration (Karschin, et. al). T/°C = 145.4845 - 39.1533Δδ - 36.062(Δδ)2 + 11.4869(Δδ)3 - 2.434(Δδ)4
Methanol-d4: N. Karschin, S. Krenek, D. Heyer, C. Griesinger "Extension and improvement of the methanol-d4 NMR thermometer calibration" Magn. Reson. Chem., 60, 185-270 (2022). T/°C = 143.3245 - 39.5133Δδ - 36.062(Δδ)2 + 11.4869(Δδ)3 - 2.434(Δδ)4
Deuterium spectrum of Methanol-d4 was measured by comparson with the proton spectrum. T/°C = 168.0279 - 99.0784Δδ + 13.2869(Δδ)2 - 6.5438(Δδ)3
The accuracy of the published data is not well determined above 60°C. At lower temperatures methanol-d4 may be as accurate as 0.03°C. Apart from this, the precision based on goodness of fit to a curve. All accuracies are for 1 standard deviation.