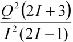Explanation of nucleus properties
Explanation of nuclear properties
Spin - The spin quantum number of the nucleus. Spin ½ nuclei generally yield sharp signals. Nuclei with higher spins are quadrupolar. The broadness of their signals increases as a function of the quadrupolar moment and asymmetry of environment. An indication of the amount of broadening is given by the linewidth factor (see below). This broadening, especially in asymmetric environments, often yields signals that are too wide to be detected with a high-resolution NMR spectrometer. These effects are not related to broadening caused by paramagnetism that may also make signals unobservable.
Natural abundance - Average abundance of this isotope as found in nature on Earth.
Chemical shift range - The chemical shift range is for the vast majority of compounds. Occasionally signals will be observed outside this range.
Frequency ratio – This is used for chemical shift referencing and is the ratio (symbol Ξ, Greek letter Xsi) of the frequency at zero ppm for this nucleus relative to the proton frequency of dilute TMS in CDCl3 as defined by IUPAC [R. K. Harris, et al., Pure Appl. Chem., 73, 1795 (2001)].
Reference compound - Compound that was used as a chemical shift reference to represent zero ppm for this nucleus prior to the IUPAC definition. If a chemical shift is given, it was measured in our laboratory relative to the IUPAC definition.
Linewidth of referenc - Width at half height of the reference compound at room temperature in a 400 MHz spectrometer.
T1 of reference - Longitudinal relaxation time of the reference compound at room temperature in a 400 MHz spectrometer.
Receptivity rel. to 1H at natural abundance - The receptivity (sensitivity) of the nucleus at natural abundance relative to the receptivity of 1H.
Receptivity rel. to 1H when enriched - The receptivity (sensitivity) of the nucleus at 100% abundance relative to the receptivity of 1H.
Receptivity rel. to 13C at natural abundance - The receptivity (sensitivity) of the nucleus at natural abundance relative to the receptivity of 13C at its natural abundance of 1.108%.
Receptivity rel. to 13C when enriched - The receptivity (sensitivity) of the nucleus at 100% abundance relative to the receptivity of 13C at its natural abundance of 1.108%.
Linewidth factor - An indication of the amount of broadening is given by the linewidth factor (in fm4) that is related to the quadrupolar moment according to equation 1 where I is the spin quantum number and Q is the quadrupole moment (in fm2). This parameter is zero for spin ½ nuclei and is omitted from their tables. See R. K. Harris and B. E. Mann, Eds., NMR and the Periodic Table, Academic Press, New York, 1978.
Equation 1.