Heteronuclear coupling
Spin-spin coupling takes place between all NMR active nuclei, not just between protons. Here examples are shown of coupling to 13C, 2D, 31P, 19F and 29Si are shown but many other nuclei can couple.
Coupling of 1H to 13C
13C has a natural abundance just over 1% and the major isotope (12C) is not NMR active so very little of the proton signal is coupled. The coupled signal appears as small satellite signals either side of the main uncoupled signal (fig. 1). One bond coupling constants are between 115 and 250 Hz (usually 125 to 160 Hz).
Fig. 1. 1H Signal of CHCl3 showing 13C coupling

carbon satellite signals usually maintain the coupling pattern of the main peak (figs. 2, 3).
Fig. 2. Main signal of a 1H AX3 multiplet

Fig. 3. 13C satellites of a 1H AX3 multiplet

Sometimes the 13C breaks the symmetry of the molecule because its neighboring atom is 12C and the multiplet structure is changed in the carbon satellites. In the case of dioxane (below) the singlet (fig. 4) becomes a complex multiplet in the satellites (fig. 5).
Fig. 4. Main signal of dioxane is a singlet

Fig. 5. 13C satellites of dioxane

In the 13C spectrum the major splittings indicate the number of attached protons, a singlet for no protons an AX doublet for one (fig. 6), AX2 for two and AX3 for three (fig. 7). Additional smaller splittings are observed arising from long-range couplings.
Fig. 6. Aromatic region of 13C spectrum of ethylbenzene showing 1H coupling

Fig. 7. Aliphatic region of 13C spectrum of ethylbenzene showing 1H coupling

Coupling of 1H and 13C to 2D
Coupling between proton and deuterium is most commonly seen in the solvent signals of the 1H spectrum (fig. 8) and sometimes in the residual water signal that has become partially deuterated. (In the case of D2O as a solvent, fast proton/deuteron exchange precludes the observation of 1H-2D coupling in the water signal.) Deuterium is a spin-1 nucleus so its coupling forms 1:1:1 triplets for each coupled deuteron, 1:2:3:2:1 quintets for two coupled deuterons and 1:3:6:7:6:3:1 septets. 1H-2D couplings are typically 1 to 2 Hz and 2D-13C couplings are typically 20 to 25 Hz.
Fig. 8. 1H-2D and 13C-2D coupling patterns typical of solvent and residual water signals

Proton coupling can also be observed in the deuterium spectrum (fig. 9). As 1H is a spin-½ nucleus, it couples to deuterium in the usual manner, forming a doublet for a single proton.
Fig. 9. 2D-NMR spectrum of CHDCl2 showing a 1.1 Hz coupling of deuterium to proton

Coupling of 1H to 31P
Long-range couplings to 31P (phosphorus) appear in the 1H spectrum of phosphorus containing molecules (fig. 10). The couplings have effects over three to four bonds. The 31P spectrum also shows 1H couplings (fig. 11). Couplings to phosphorus are also observed in the 13C spectrum (not shown here).
Fig. 10. 1H spectrum of sphingomyelin showing the effects of 31P coupling

Fig. 11. 31P spectrum of sphingomyelin showing the effects of 1H coupling

Coupling of 1H and 13C to 19F
Fluorine couples strongly to proton up to three bonds away (fig. 12). This can bee seen as extra splittings in the 1H spectrum.
Fig. 12. 1H spectrum of 2-fluoro-2-deoxyglucose showing 19F coupling

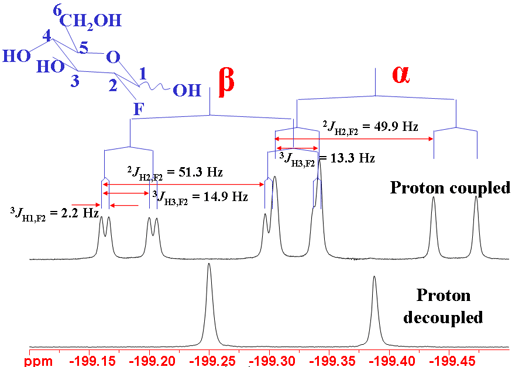
The coupling patterns are clear in the 19F spectrum (fig. 13).
Fig. 13. 19F spectrum of 2-fluoro-2-deoxyglucose showing 1H coupling
Fluorine couples strongly to 13C up to three bonds away (fig. 14). This can bee seen as extra splittings in the 13C spectrum
Fig. 14. 13C spectrum of 2-fluoro-2-deoxyglucose showing 19F coupling

Coupling of 1H to 29Si
Proton silicon coupling is most commonly observed in the TMS signal (fig. 15) but is observed in other silyl compounds. A two bond coupling of 6.6 Hz is typical and the natural abundance of less than 5% for 29Si means that its coupling appears as satellites.
Fig. 15. 1H signal of TMS showing 13C and 29Si couplings as satellite signals
