13Carbon NMR
Use our NMR service that provides 13C NMR and many other NMR techniques.
The 1D 13Carbon NMR experiment is much less sensitive than Proton (1H) but has a much larger chemical shift range. Its low natural abundance (1.108%) and proton decoupling means that spin-spin couplings are seldom observed. This greatly simplifies the spectrum and makes it less crowded. 13C is a low sensitivity nucleus that yields sharp signals and has a wide chemical shift range.
A typical analysis of a 13C NMR spectrum consists of matching expected chemical shifts to the expected moieties. Our NMR service provides 13C NMR along with many other NMR techniques. Each type of signal has a characteristic chemical shift range that can be used for assignment (fig. 1).
Fig. 1. Chemical shift ranges of carbons according to their chemical environment
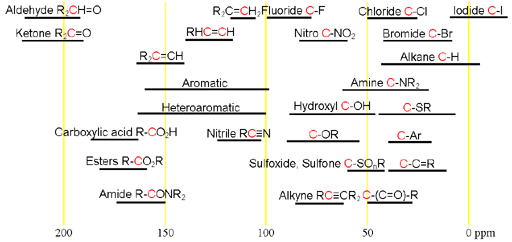
Choose the structure that most closely represents the hydrogen in question. R = alkyl or H, Ar = aryl.
Integration is almost useless in a regular 13C NMR spectrum because of uneven nuclear Overhauser effect (NOE) enhancement of the signals by decoupling and long longitudinal relaxation times (T1's). Quantitative spectra may be obtained by inverse gated decoupling and long delays in the region of 10 minutes between pulses. However, this is very insensitive for 13C and is rarely a realistic option. Fig. 2 shows the signal enhancement due to NOE of the decoupled spectrum of ethylbenzene under comparable conditions to the quantitative spectrum. The enhancement is much greater under routine conditions where much shorter repetition times and sensitivity enhancing window functions are used.
Fig. 2. NOE enhancement in a 13C NMR spectrum
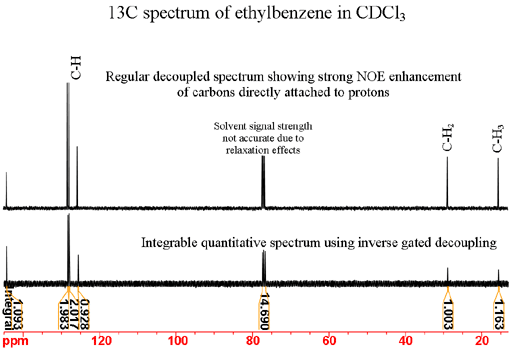
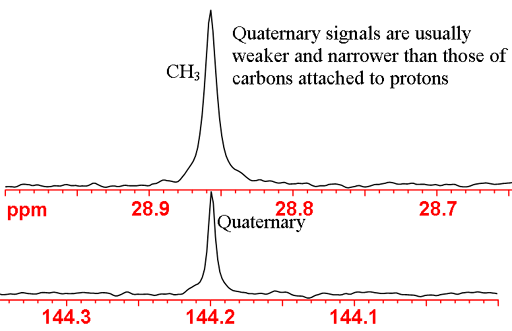
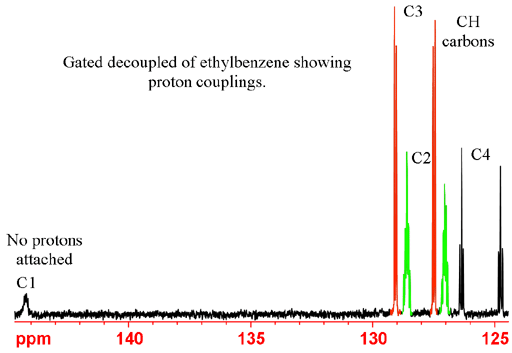
The chemical shifts are used for the initial assignment of the spectrum. For ethylbenzene, we see that the signals at 15.6 and 28.9 ppm fall in the aliphatic region and therefore belong to the CH2 and CH3 carbons. Usually CH3 has a lower chemical shift than CH2 so can be provisionally assigned to 15.6 and 28.9 ppm, respectively. The remaining signals are in the aromatic region at 125.6, 127.8, 128.3 and 144.2. The carbon not attached to any protons is called 'quaternary' (four-fold) even though this is a misnomer for unsaturated carbons such as in our case where it is only attached to only three other carbons. Quaternary carbons usually give sharper signals than other carbons and usually give weaker signals (fig. 3) under normal acquisition conditions – decoupling and relatively short repetition times. This is because of their slow relaxation and lack of NOE enhancement. The chemical shifts of aromatic carbons not attached to protons (for ethylbenzene C1) are generally higher than for those attached (for ethylbenzene C2, C3 and C4). Therefore, the signal at 144.2 ppm is provisionally assigned to C1.
Fig. 3. Differentiation of quaternary from proton-attached carbons using NOE enhancement
Two pairs of carbons (C2, C6 and C3, C5) have chemical shift equivalence and there have an integral of 2 in the quantitative spectrum while C4 has an integral of 1. All three signals are of type CH so their line-widths and their NOE enhancement upon decoupling are similar. As a result, the ratio of 2:2:1 in peak heights is fairly well preserved in the regular decoupled spectrum and can be used to assign the signal at 125.6 ppm to C4.
If 2D-NMR experiments are to be used to achieve a full assignment then no further 1D experiments are needed. However, if the molecule is simple or only a partial assignment is required then one or more further 1D experiments such as gated decoupling, APT and DEPT may be worthwhile.
If the 13C spectrum is not sensitive enough, consider using the projection of a heteronuclear correlation (short range and long range) to improve sensitivity. If having combined the 13C data with the 1H NMR data it is still not possible to analyze the spectrum to your satisfaction, then other NMR techniques such as 2D NMR are required.
Gated decoupled 13C NMR
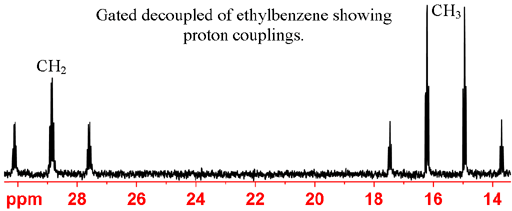
Gated decoupling may be used in order to observe proton couplings. Gated decoupling is preferable to no decoupling because it preserves the NOE sensitivity enhancement. In the coupled spectrum (fig. 4) methine (CH) carbons appear as doublets, methylene (CH2) carbons as 1:2:1 triplets and methyl (CH3) carbons as 1:3:3:1 quartets.
Fig. 4. Gated decoupled 13C spectrum of ethylbenzene showing CH3 quartet, CH2 triplet, CH doublets and a carbon that has only long-range proton couplings.
The coupling constants are typically 125 Hz for sp3 carbons, 160 Hz for sp2 carbons and
200 Hz for sp carbons. However, electronegative substituents increase the coupling constants, e.g., 214 Hz
for chloroform, while electropositive ones reduce the coupling, e.g., 117 Hz for TMS. The one-bond coupling
constant can be used as a measure of hybridization when there are no strongly electropositive or electronegative
substituents. Long range couplings (predominantly 3-bond that are usually paradoxically stronger than 2-bond) of
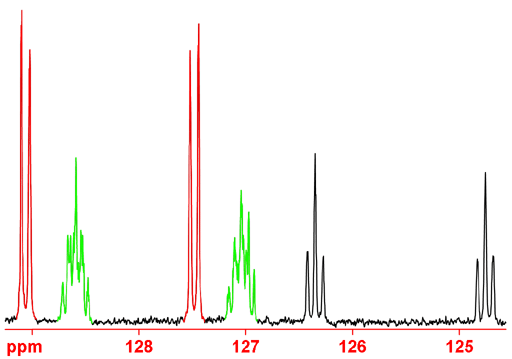
up to about 10 Hz can be observed but may be difficult to assign. In the case of ethylbenzene (fig. 5), the long-range
couplings can be used to assign the aromatic CH carbons. C4 can be assigned using the intensity arguments above.
The long-range couplings visible in the gated decoupled experiment are mostly three-bond (this is usually but not
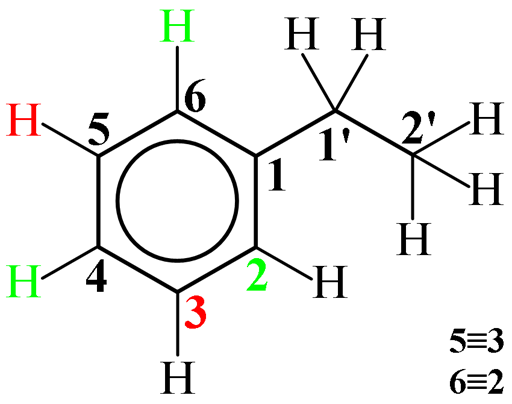
always the case) and can be used to differentiate C2 and C3. In the diagram below, C3 (and its coupled protons in
red) is coupled to one proton and is therefore a double doublet in the gated decoupled
spectrum. C2 (and its coupled protons in green) would be expected to be a double
triplet but is more complex, due to second order coupling
between protons. Three-bond couplings display a Karplus type relation
( in Hz) with torsion angle, θ, and can sometimes
be used to estimate the torsion angle. Couplings may be observed with other
nuclei such as 19Fluorine or 31Phosphorus.
in Hz) with torsion angle, θ, and can sometimes
be used to estimate the torsion angle. Couplings may be observed with other
nuclei such as 19Fluorine or 31Phosphorus.
Fig. 5. Ethylbenzene showing three-bond proton couplings to carbons 2 and 3.
Attached proton test (APT)
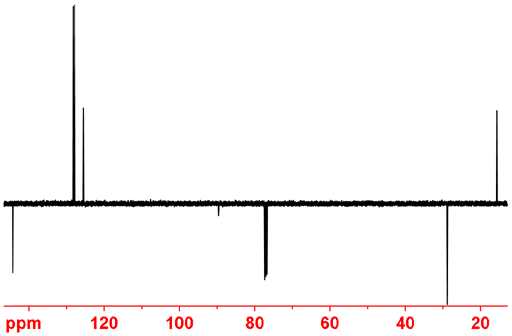
However, if only multiplicity is important rather than coupling constant then the attached proton test (APT) and distortionless enhancement by polarization transfer (DEPT) experiments are more sensitive than gated decoupling. The APT experiment yields methine (CH) and methyl (CH3) signals positive and quaternary (C) and methylene (CH2) signals negative (fig. 6). It is slightly less sensitive than DEPT but shows all carbon signals at once unlike DEPT that suppresses quaternary carbons.
Fig. 6. APT spectrum of ethylbenzene showing CH and CH3 positive while CH2 and C are negative.
DEPT
The DEPT experiment is slightly more sensitive than APT and can fully separate the carbon signals. However, it has to be run three times with different final pulse angles and compared with the regular decoupled 13C spectrum in order to provide a full analysis. The DEPT experiment requires at least four scans in order to cancel out the quaternary signals although many more scans are usually acquired so this is not a problem. DEPT 45 (fig. 7) yields CH, CH2 and CH3 signals positive, DEPT 90 (fig. 8) yields only CH signals and DEPT 135 (fig. 9) yields CH and CH3 positive while CH2 s negative.
Fig. 7. DEPT 45 spectrum of ethylbenzene showing only carbons attached to protons: CH, CH2 and CH3 all positive.

Fig. 8. DEPT 90 spectrum of ethylbenzene showing only CH carbons. Suppression of attached CH2 and CH3 is not complete.
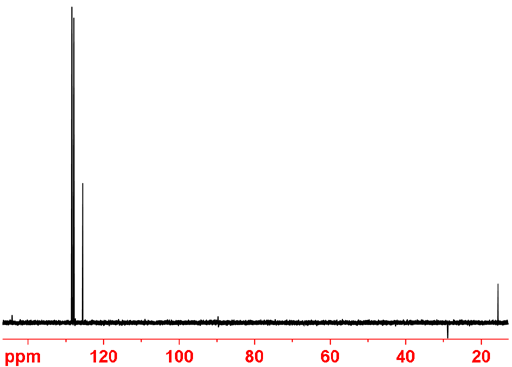
Fig. 9. DEPT 135 spectrum of ethylbenzene showing only CH, and CH3 positive and CH2 negative.
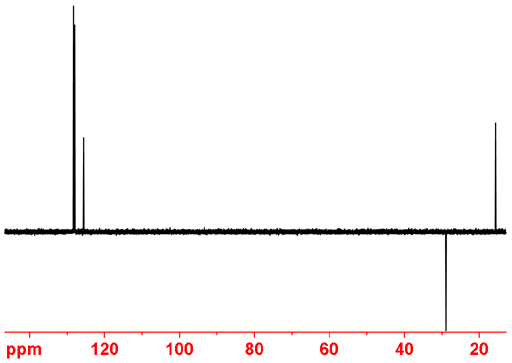
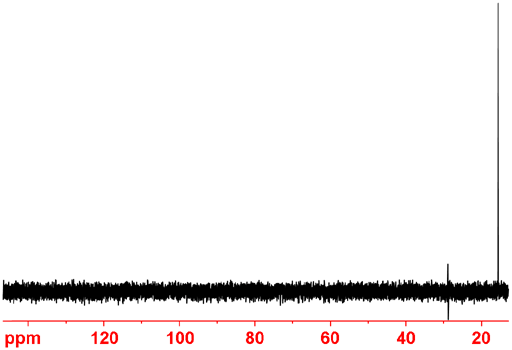
In theory, adding DEPT 45 – 0.08 DEPT 90 - 1.2 DEPT 135 yields CH2 only (fig. 10) and DEPT 45 -1.52 DEPT 90 + 1.2 DEPT 135 yields CH3 only (fig. 11). However, slight adjustments to these factors are required by trial and error in practice. In principle, subtracting DEPT 45 from the regular spectrum yields the quaternary carbons only. However, suppression is poor because of slight changes in temperature of the sample due to decoupling that shift the peaks slightly.
Fig. 10. Combination of DEPT spectra of ethylbenzene showing only CH2.

Fig. 11. Combination of DEPT spectra of ethylbenzene showing only CH3.
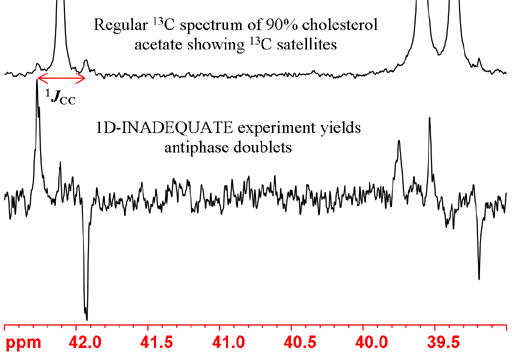
Carbon-carbon couplings
Couplings to other carbons yield very weak signals at natural abundance and appear as small satellites in very concentrated samples or larger satellites in 13C-enriched samples. The one-bond coupling constants can be used to estimate carbon-carbon bond order. The constants are usually 35 to 45 for a single bond and about 65 Hz for a double bond and are increased by electronegative substituents. Often the peak overlap obscures the 13C satellites (as in the right-hand peaks in fig. 12) in which case the coupling constants can be measured using a 1D-INADEQUATE (incredible natural abundance double quantum transfer experiment). INADEQUATE suppresses the main uncoupled signal and yields only the satellites as antiphase (one up and one down) multiplets. However, INADEQUATE requires enriched or very concentrated samples and careful pulse calibration. In principle, the INADEQUATE experiment could be used for assignment by comparing coupling constants and looking for AB type roofing effects, but unless there are no protons near the carbons, there are much more sensitive assignment techniques available.
Fig. 12. 13C spectra showing 13C couplings.
Properties of 13C
| Property | Value |
|---|---|
| Spin | ½ |
| Natural abundance | 1.108% |
| Chemical shift range | 200 ppm, from 0 to 200 |
| Frequency ratio (Ξ) | 25.145020% |
| Reference compound | TMS < 1% in CDCl3 = 0 ppm |
| Linewidth of reference | 0.19 Hz |
| T1 of reference | 9 s |
| Receptivity rel. to 1H at natural abundance | 1.70×10-4 |
| Receptivity rel. to 1H when enriched | 0.0159 |
| Receptivity rel. to 13C at natural abundance | 1.00 |
| Receptivity rel. to 13C when enriched | 93.5 |
Safety note:
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, TMS is toxic, volatile and flammable: wear protective gloves and work in a hood. All deuterated compounds are toxic.