Distortionless enhancement by polarization transfer (DEPT)
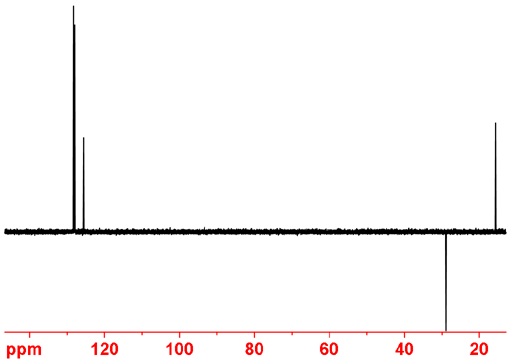
The distortionless enhancement by polarization transfer (DEPT) experiment is slighlty more sensitive than APT and can fully separate the carbon signals. However, it has to be run three times with different final pulse angles and compared with the regular decoupled 13C spectrum in order to provide a full analysis. The DEPT experiment requires at least four scans in order to cancel out the quaternary signals although many more scans are usually acquired so this is not a problem. DEPT 45 yields CH, CH2 and CH3 signals positive, DEPT 90 yields only CH signals and DEPT 135 yields CH and CH3 positive while CH2 is negative. The use of DEPT in 13C assignment is described in connection with 13C NMR. Fig. 1 shows the APT spectrum of ethylbenzene.
Fig. 1. DEPT 135 spectrum of ethylbenzene showing CH and CH3 positive and CH2 negative
The APT pulse sequence is shown in fig. 2. The delay between the first two pulses is set to one over twice the carbon proton coupling constant. For CH2 (which is the deciding factor for this experiment) this is usually about 125 Hz so the delay is 4 ms. Between the last two pulses and between the last pulse and the acquisition 100μs is usually sufficient.
Fig. 2. Pulse sequence for DEPT