NMR chromatography
We are the only laboratory in the World offering NMR chromatography service.
Why is NMR chromatography needed?
NMR spectroscopy is an excellent method and therefore usually a tool of choice for precise structural characterization of organic and bio-molecules. NMR is most suited to the analysis of pure compounds. However, NMR spectroscopy is limited and almost useless when several compounds of various structures are present in the solution mixture. Most organic syntheses initially yield a mixture of compounds that require a separation process prior to structural characterization by NMR, if indeed separation is possible.
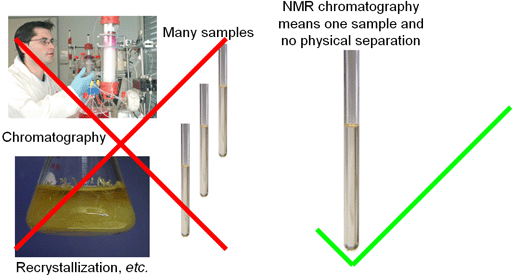
NMR chromatography, resolves mixtures into their components spectroscopically, giving separate spectra for each component, all in a few minutes (fig. 1). This does away with the need for physical separation of the sample, saving time, effort and money.
Use our NMR service for NMR chromatography.
Fig. 1. NMR chromatography saves time and money by doing away with physical separation of components
What you should already know before continuing to read this:
Before you continue reading about diffusion NMR, you should have some knowledge of:
If you want to read about these subjects first, please go to the links above.
How can NMR chromatography help?
To a certain extent, 2D and multidimensional NMR can separate simple mixtures but a more suitable method is to separate the components according to their diffusion coefficients. This is achieved with pulsed magnetic gradients using self-diffusion (SD) NMR techniques, also known, when presented as a 2D contour map, as Diffusion Ordered SpectroscopY (DOSY). The chemical shift of the spectrum is usually plotted along the horizontal axis while the diffusion rate is plotted on a perpendicular axis. Each component yields a separate regular 1D spectrum corresponding to its diffusion constant. This application of DOSY has been dubbed NMR chromatography (fig. 1). In recent reports NMR chromatography has been used to refer to DOSY spectra where structured media such as silica gel was used to enhance the separation in the diffusion dimension. The NMR chromatography technique is not true classical chromatography because there is no phase possessing mean movement. Although the term "NMR chromatography" has been used in several papers, it may be better to term this technique pseudochromatographic NMR.
There also exist hyphenated techniques such as LC-NMR where the mixture is separated by conventional chromatography and NMR is then automatically applied to each fraction. However these methods require specialist equipment, are more expensive and are less sensitive than NMR chromatography.
The main disadvantage with the conventional DOSY method is that in most cases there is insufficient separation in the diffusion axis to fully separate the components of the mixture. The free induction decay (FID) of a DOSY experiment is a sum of decaying sinusoids in the acquisition dimension and a sum of Gaussian decays in the diffusion dimension. The acquisition dimension is easily analyzed by a Fourier transform yielding high resolution in the frequency domain. However, analysis of the diffusion dimension involves an inversion of the Laplace transform (ILT). While this is quite accurate at up to 2% for a single decay, it has very low resolution when separating two or more overlapping signals with little chance of resolving diffusions of signals that have overlapping frequencies that differ by less than 30–50%.
Attempts to enhance the separation in the diffusion dimension were made by adding a solid chromatographic medium such as silica gel. In conventional chromatography, the silica gel differentially binds each compound, giving each compound a different translational velocity in the column. Likewise, there is a differentiation in the compounds' effective diffusion rates even though there is no flow. However, using conventional NMR techniques solid silica gel broadens the signals arising from the liquid to hundreds or thousands of Hertz due to inhomogeneous magnetic susceptibility. Signals arising from solids that tumble slowly if at all are broadened to tens of kHz due to chemical shift anisotropy. DOSY requires narrow signals in 1D NMR in order to yield signals in a diffusion spectrum. This is because broad signals relax almost completely during the diffusion pulse sequence prior to acquisition. Therefore, solid-state NMR techniques such as high resolution magic angle spinning (HR-MAS) or susceptibility matching of the solvent to the silica have been used to observe the spectrum. This greatly complicates the application of this technique and provides limited results.
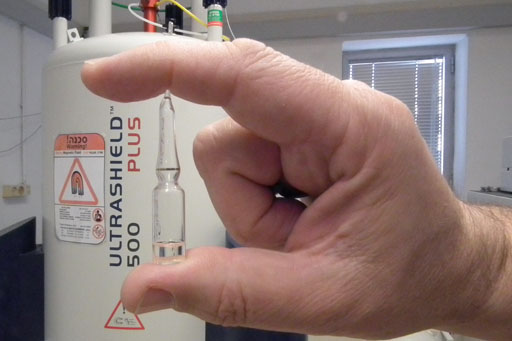
The advantages of our patent liquid nanotechnology (fig. 2) over silica for NMR chromatography are:
- A regular NMR spectrometer for liquid-state is all that is needed without an HR-MAS probe or magic angle spinning capability.
- There is no need for high-susceptibility solvents that are usually brominated or iodated and therefore, very photosensitive.
- The sample is measured without spinning so the diffusion rate is not affected by spinning.
- Our liquid nanotechnology can be stable for years so there is no need to prepare the 'solvent' immediately prior to use.
Fig. 2. Ampoule of an NMR chromatography 'solvent'
Applications of NMR chromatography
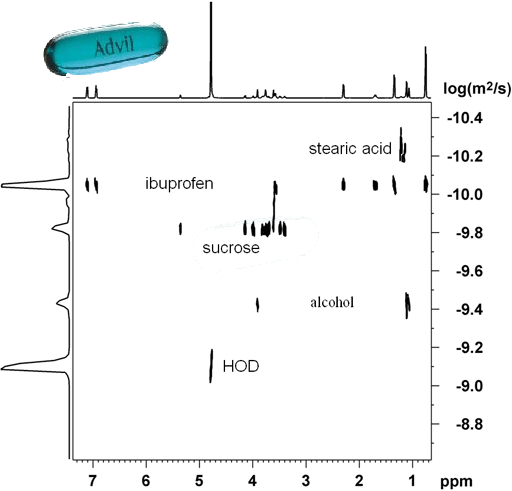
A regular diffusion or DOSY experiment usually gives limited separation in the diffusion domain as shown in fig. 3 for the pharmaceutical drug, Advil, in D2O. The application of an NMR chromatography solvent transforms the DOSY spectrum into something much more useful that clearly separates all the components of the formulation (fig. 4).
Fig. 3. The DOSY spectrum of the pharmaceutical drug, Advil, shows little separation in the diffusion (vertical) direction
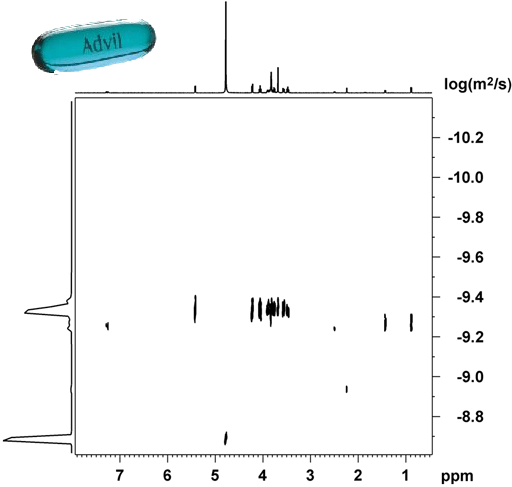
Fig. 4. Separation of the pharmaceutical drug, Advil, into its components by NMR chromatography
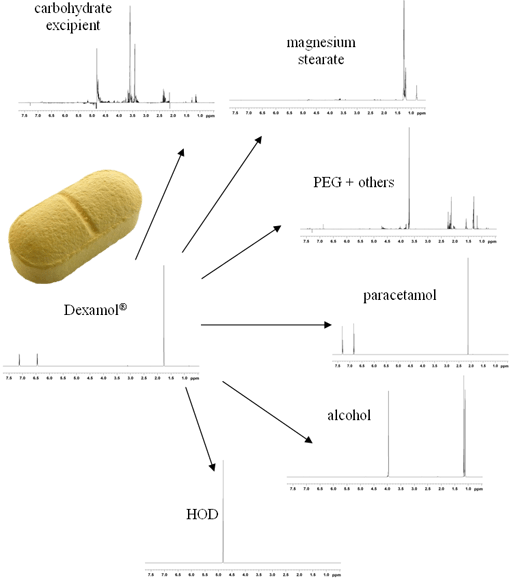
NMR chromatography has been applied to a variety of mixtures including pharmaceutical drugs (fig. 5) and reaction mixtures. The 1H NMR spectrum is separated into separate spectra (fig. 5) for each component of the mixture.
Fig. 5. Separation of the pharmaceutical drug, Dexamol, into its components by NMR chromatography
3D and higher dimensional DOSY can be applied to NMR chromatography by combining 2D techniques such as TOCSY with the DOSY experiment. Fig. 6 shows the 3D DOSY-TOCSY spectrum of Dexamol in an NMR chromatography solvent.
Fig. 6. 3D DOSY-TOCSY NMR chromatography spectrum of Dexamol
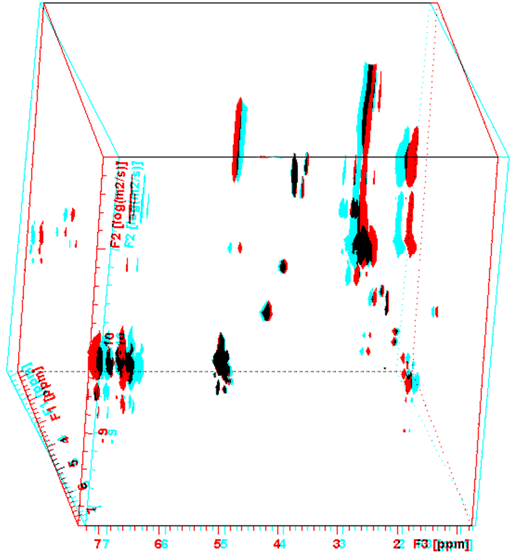
To see this figure correctly place a red filter of the left eye and a cyan filter over the right eye.
Literature references
- P. Hodge, P. Monvisade, G. A. Morris and I. Preece, "A novel NMR method for screening soluble compound libraries", Chem. Commun., 239-240 (2001).
- S. Caldarelli, "Procede d'analyse utilisant la RMN", Fr. Pat. 2847674 (2002).
- S. Viel, F. Ziarelli and S. Caldarelli, "Enhanced diffusion-edited NMR spectroscopy of mixtures using chromatographic stationary phases", PNAS, 100, 9696–9698 (2003).
- G. Pages, C. Delaurent, S. Caldarelli, "Investigation of the chromatographic process via pulsed-gradient spin-echo nuclear magnetic resonance. Role of the solvent composition in partitioning chromatography", Anal. Chem., 78 561–566 (2006).
- S. Caldarelli, "Chromatographic NMR: a tool for the analysis of mixtures of small molecules" Magn. Reson. Chem., 45, S48 (2007).
- R. E. Hoffman, A. Aserin, N. Garti, "System and methods for diffusion ordered spectroscopy", PCT Int. Appl. WO 2008050347 A2 (2008).
- R. E. Hoffman, H. Arzuan, C. Pemberton, A. Aserin and N. Garti, "High-resolution NMR "chromatography" using a liquids spectrometer", J. Magn. Reson., 194, 265 (2008).
- C. Carrara, S. Viel, F. Ziarelli, G. Excoffier, C. Delaurent and S. Caldarelli, "Chromatographic NMR in NMR solvents", J. Magn. Reson., 194, 303-306 (2008).
- J. S. Kavakka, I. Kilpeläinen, and S. Heikkinen, "General Chromatographic NMR Method in Liquid State for Synthetic Chemistry: Polyvinylpyrrolidone Assisted DOSY Experiments", Org. Lett., 11, 1349-1352 (2009).
- R. E. Hoffman, C. Pemberton, A. Aserin, N. Garti, "NMR Chromatography using nano-structured liquids", PCT Int. Appl., WO 2010023673 (2010).
- R. E. Hoffman and R. Shenhar, "Polymer, copolymer and oligomers and uses thereof for NMR Chromatography in homogeneous solutions", patent pending (2010).
- C. Pemberton, R. E. Hoffman, A. Aserin and N. Garti, "New insights into silica based NMR "Chromatography"", J. Magn. Reson., in press (2011).
- C. Pemberton, R. E. Hoffman, A. Aserin and N. Garti, "NMR chromatography using ...", in preparation.
- C. Pemberton, "NMR chromatography using silica and dispersed systems", Thesis to the Hebrew University in preparation.