TOCSY
1H-1H TOCSY (TOtal Correlated SpectroscopY also known as HOHAHA – HOmonuclear HArtmann HAhn) is useful for dividing the proton signals into groups or coupling networks, especially when the multiplets overlap (have very similar chemical shifts) or there is extensive second order coupling. A TOCSY spectrum yields through bond correlations via spin-spin coupling. Correlations are seen throughout the coupling network and intensity is not related in a simple fashion to the number of bonds connecting the protons. Therefore a five-bond correlation may or may not be stronger than a three-bond correlation. TOCSY is usually used in large molecules with many separated coupling networks such as peptides, proteins, oligosaccharides and polysaccharides. If an indication of the number of bonds connecting the protons is required, for example in order to determine the order in which they are connected, a COSY spectrum is preferable.
The pulse sequence (fig. 1) used in our laboratory is the gradient enhanced TOCSY that means that during the pulse sequence, magnetic field gradients are applied. The spin-lock is a composite pulse and should be applied for between 20 and 200 ms with a pulse power sufficient to cover the spectral width. A short spin-lock makes the TOCSY more COSY-like in that more distant correlations will usually be weaker than short-range ones. A long spin-lock holds the magnetic vector in the x-y plane allowing correlations over large coupling networks. The length of the spin-lock is roughly related to the distance through the coupling network that correlations are seen. However, too long a spin-lock will heat the sample causing signal distortion and can damage the electronics of the spectrometer. Therefore care should be taken.
The attenuation should be set so that the 90° pulse width will be less than 1/(4SWH) (SWH is the spectral width in Hz) and typically 1/(6SWH). An attenuation of 12 dB with a 50 W amplifier yielding a 90° pulse width of 35 μs is typical for a 5 mm high resolution probe.
Fig. 1. Pulse sequence for gradient TOCSY
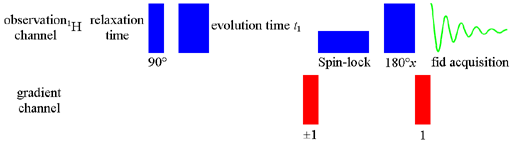
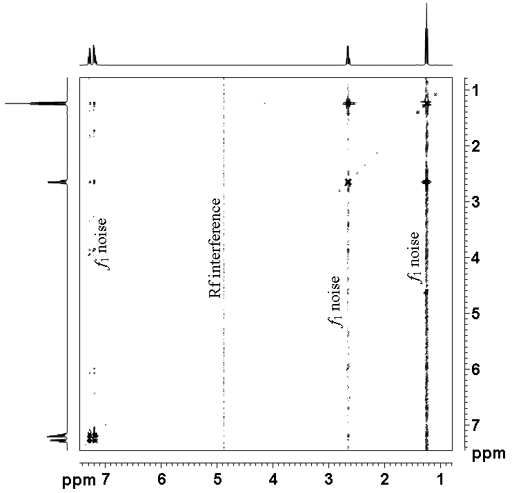
The TOCSY spectrum as shown in fig. 2 for ethylbenzene (fig. 3) contains a diagonal and cross-peaks. The diagonal consists of the 1D spectrum with single peaks suppressed. The cross-peaks indicate couplings between two mutliplets. The cross-peaks correspond to correlations through bonds, the most apparent of these is between H1' and H2' at 2.65 and 1.24 ppm. All the desired signals (that correspond to connectivity) are pure phase, albeit with minor symmetrical phase distortion. In addition, artifacts (undesired signals) also appear in the spectrum as vertical streaks (interference and f1 noise, fig. 4). These artifacts are rarely in phase with the desired signals and appear in specific locations.
Fig. 2. 2D TOCSY spectrum of ethylbenzene
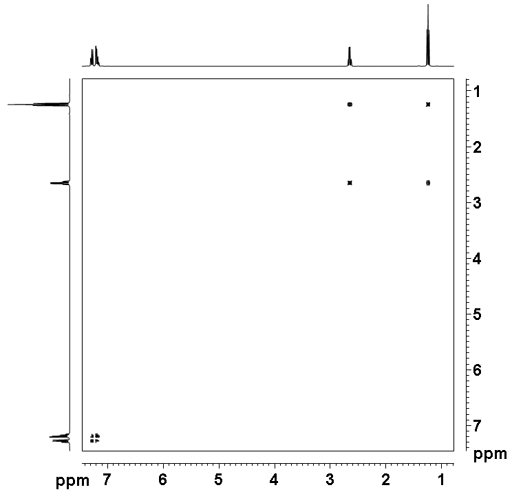
Fig. 3. Structure of ethylbenzene
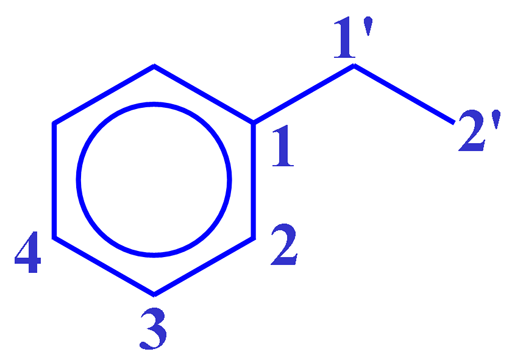
Fig. 4. Artifacts in the TOCSY spectrum of ethylbenzene
For example, in 12,14-ditbutylbenzo[g]chrysene (fig. 5), only a partial analysis of the regular 1H-NMR spectrum is possible. TOCSY (fig. 6) confirms the initial division of protons intofour groups or coupling networks. No correlations (cross-peaks) are seen to the tbutyls (at 0.92 and 1.40 ppm) because they are too many bonds away from the ring system.
Fig. 5. Structure of 12,14-ditbutylbenzo[g]chrysene
![12,14-ditbutylbenzo[g]chrysene](tocsy_files/dtbbgc.gif)
Fig. 6. Artifacts in the COSY spectrum of ethylbenzene
![TOCSY of 12,14-ditbutylbenzo[g]chrysene](tocsy_files/tocsydtbbgc.gif)
The aromatic region of the spectrum (fig. 7) shows all the correlations within each coupling network. However, unlike COSY, these cannot be used to determine which protons are neighbors. For example the proton at 8.62 ppm is next to the proton at 7.55 ppm but its correlation is weaker than with the four-bond correlation to the signal at 7.59 ppm which is in turn weak than the five-bond correlation to the proton at 8.56 ppm.
Fig. 7. Aromaric region of the 2D TOCSY spectrum of 12,14-ditbutylbenzo[g]chrysene
![Aromatic region of TOCSY of
12,14-ditbutylbenzo[g]chrysene](tocsy_files/aromatic.gif)
Using horizontal and vertical lines, it is possible to separate each group and follow its connectivity (fig, 8). The blue group of four protons consist of the signals at 8.62 ppm, 7.55, 7.59 to 8.56, the green group of four protons at 8.54 to 7.34 to 7.44 to 8.17 and the red group or two protons, that correspond to H9 and 10 because they are the only group of two protons expected to have a three-bond coupling constant (8.9 Hz), are at 7.76 and 8.32 ppm. The yellow group of two protons correspond to H11 and 13 because the coupling constant is small (1.9 Hz) and consistent with a four bond correlation.
Fig. 8. Aromaric region of the 2D TOCSY spectrum of 12,14-ditbutylbenzo[g]chrysene showing connectivity and separation into four color-coded proton groups
![Aromatic region of TOCSY of
12,14-ditbutylbenzo[g]chrysene in color](tocsy_files/aromaticcolor.gif)