(Sn) Tin NMR
Use our NMR service that provides Sn NMR and many other NMR techniques.
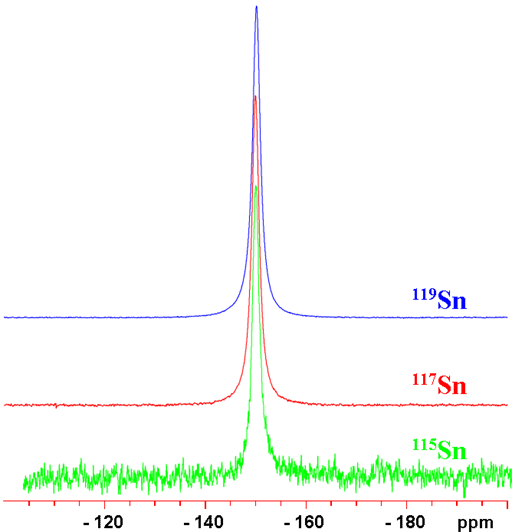
Tin is unique in that it has no less than three NMR active spin ½ nuclei, 115Sn, 117Sn and 119Sn, that yield narrow signals (fig. 1) over a very wide chemical shift range. 119Sn is very slightly the more sensitive than 117Sn so 119Sn is therefore usually the preferred nucleus. 115Sn is much less sensitive than either 117Sn or 119Sn. Tin NMR is mostly used for the study of organotin compounds, but is also applicable to inorganic tin compounds.
Fig. 1. Comparison of the NMR spectra of the tin isotopes 115Sn, 117Sn and 119Sn for SnCl4 (neat)
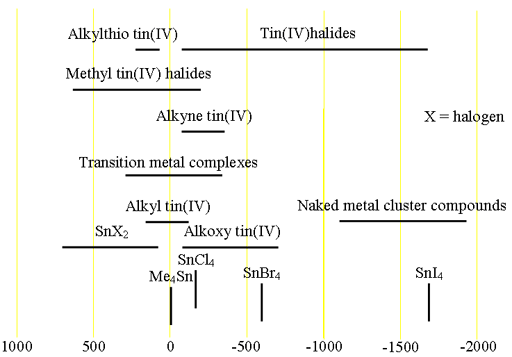
Each type of tin compound has its characteristic chemical shift range (fig. 2).
Fig. 2. Chemical shift ranges for tin NMR
All the tin nuclei couple to other nuclei, 1H, 13C, 19F, 31P, etc., couplings have been reported. One bond couplings to 13C are getween 1200 and 1500 Hz. To 1H one bond couplings are from 1750 to 3000 Hz, to 19F from 130 to 2000 Hz and to 31P they are from 50 to 2400 Hz. Two-bond Sn-H coupling constants are apporximately 50 Hz. Homonuclear 119Sn-119Sn and heteronuclear 119Sn-117Sn have been reported from 200 to 4500 Hz. Three and four bond couplings have been reported.
115Tin NMR
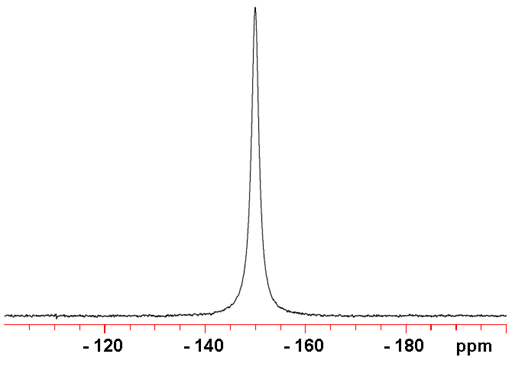
115Sn is much less sensitive than the other tin nuclei, so is not the preferred nucleus of tin. It is a spin ½ nucleus and yields sharp signals (fig. 3).
Fig. 3. 115Sn-NMR spectrum of SnCl4 (neat)

Properties of 115Sn
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 0.34% |
| Chemical shift range | 2600 ppm, from -1900 to 700 |
| Frequency ratio (Ξ) | 32.718749% |
| Reference compound | Me4Sn 90% in C6D6 |
| Linewidth of reference | 2.7 Hz |
| T1 of reference | 0.65 s |
| Receptivity rel. to 1H at natural abundance | 1.24 × 10-4 |
| Receptivity rel. to 1H when enriched | 0.0365 |
| Receptivity rel. to 13C at natural abundance | 0.711 |
| Receptivity rel. to 13C when enriched | 209 |
117Tin NMR
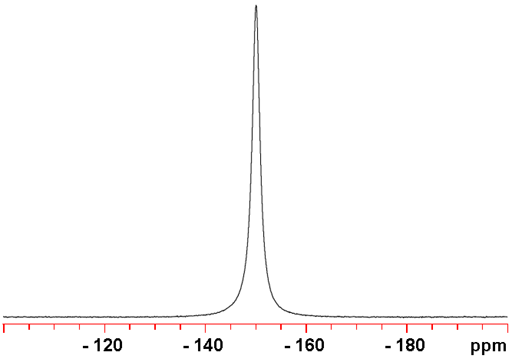
117Sn is slightly less sensitive than 119Sn, so is not usually the preferred nucleus of tin. It is a spin ½ nucleus and yields sharp signals (fig. 4).
Fig. 4. 117Sn-NMR spectrum of SnCl4 (neat)
Properties of 117Sn
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 7.68% |
| Chemical shift range | 2600 ppm, from -1900 to 700 |
| Frequency ratio (Ξ) | 35.632259% |
| Reference compound | Me4Sn 90% in C6D6 |
| Linewidth of reference | 2.5 Hz |
| T1 of reference | 0.65 s |
| Receptivity rel. to 1H at natural abundance | 3.54 × 10-3 |
| Receptivity rel. to 1H when enriched | 0.04615 |
| Receptivity rel. to 13C at natural abundance | 20.8 |
| Receptivity rel. to 13C when enriched | 271 |
119Tin NMR
119Sn is slightly more sensitive than 117Sn and much more sensitive than115Sn, so it is usually the preferred nucleus of tin. It is a spin ½ nucleus and yields sharp signals (fig. 5).
Fig. 5. 119Sn-NMR spectrum of SnCl4 (neat)
Properties of 119Sn
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 8.59% |
| Chemical shift range | 2600 ppm, from -1900 to 700 |
| Frequency ratio (Ξ) | 37.290632% |
| Reference compound | Me4Sn 90% in C6D6 |
| Linewidth of reference | 2.5 Hz |
| T1 of reference | 0.65 s |
| Receptivity rel. to 1H at natural abundance | 4.53 × 10-3 |
| Receptivity rel. to 1H when enriched | 0.0527 |
| Receptivity rel. to 13C at natural abundance | 26.6 |
| Receptivity rel. to 13C when enriched | 310 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, tin may be toxic in large doses. Tin (IV) chloride reacts violently with water yielding corrosive and poisonous HCl.
References
- D. E. Williams, L. H. Toporcer and G. M. Ronk, "Nuclear magnetic resonance coupling constants in tin in 3,3,3-trifluoropropyltin compounds", J. Phys. Chem., 74, 2139-42 (1970).
- E. V. Van den Berghe and G. P. Van der Kelen, "Study of the chemical bond in (CH3)nSn(SCH3)4-n(n=0,1,2,3) by NMR proton, tin-119, and carbon-13 spectroscopy", J. Organometal. Chem., 26, 207-13 (1971).
- J. D. Kennedy, W. McFarlane and G. S. Pyne, "Effect of interbond angles at tin upon tin-119 chemical shifts in organotin alkane-α ,ω-dithiolates and some related compounds", Bull. Soc. Chim. Belg., 84, 289-298 (1975).
- J. D. Kennedy, W. McFarlane, G. S. Pyne, P. L. Clarke and J. L. Wardell, "Magnetic double resonance studies of tin-119 chemical shifts in compounds with tin-sulfur bonds and related species", J. Chem. Soc., Perkin Trans. 2, 1234-1239 (1975).
- T. N. Mitchell, "Substituent chemical shifts of the organotin moiety in compounds of the type RSnMenCl3-n(n = 0 to 3; R = n-alkyl)", Org. Magn. Reson., 8, 34-39 (1976).
- A. Tupciauskas, "Tin-119 NMR constants for organotin compounds with remote substituents", Lietuvos Fizikos Rinkinys, 17, 233-238 (1977).
- B. Wrackmeyer, "Carbon-13 and tin-119 NMR studies on alkynylstannanes", J. Organometal. Chem., 145, 183-188 (1978).
- T. N. Mitchell, and G. Walter, "Fourier transform nuclear magnetic resonance investigations of organotin compounds. Part 6. Tin-119 and carbon-13 nuclear magnetic resonance. Spectra of hexaorganoditins and octaorganotritins", J. Chem. Soc., Perkin Trans. 2, 1842-1827 (1977).
- R. Hani, and R. A. Geanangel, "Tin-119 NMR in coordination chemistry", Coordination Chem. Rev., 44, 229-246 (1982).
- D. Dakternieks, R. W. Gable, and G. Winter, "Tin-119 and carbon-13 NMR studies on tin(IV) xanthates", Inorg. Chim. Acta, 75, 185-188 (1983).
- K. Kobayashi, M. Kawanisi, T. Hitomi and S. Kozima, "Bonding isomers of triorganostannyl enolates analyzed by tin-119 NMR spectroscopy", Chem. Lett., 497-500 (1984).
- H. Killing, H. and T. N. Mitchell, "New tin heterocycles: adducts between acetylenes or allenes and 'distannacyclopropanes'" Organometallics, 3, 1917-1919 (1984).
- A. Lycka, M. Nadvornik, K. Handlir, and J. Holecek, "Carbon-13 and tin-119 NMR spectra of some triphenyltin 4-substituted benzoates dissolved in coordinating and non-coordinating solvents", Coll. Czech. Chem. Comm., 49, 2903-2911 (1984).
- T. Yano, K. Nakashima, J. Otera, and R. Okawara, "Tin-119 NMR spectroscopic study on tetraorganodistannoxanes", Organometallics, 4, 1501-1503 (1985).
- J. Bluemel, and F. H. Koehler, "Direct and indirect metallation of endo-dicyclopentadiene. Tin-119 and carbon-13 NMR studies of stannylated derivatives", J. Organometal. Chem., 340, 303-315 (1988).
- T. N. Mitchell, J. C. Podesta, A. Ayala, and A. B. Chopa, "Experimental test of Karplus-type relationships for 3J(tin, carbon) and 3J(tin, hydrogen)", Magn. Reson. Chem., 26, 497-500 (1988).
- I. Wharf, "Studies in aryltin chemistry. Part 5. Tin-119 and carbon-13 NMR spectra of some tetra- and triaryltin compounds", Inorg. Chim. Acta, 159, 41-48 (1989).
- F. Thunecke, and R. Borsdorf, "Tin-119 NMR spectroscopic investigations of tributyltin derivatives of aromatic sulfonic acids", J. Prakt. Chem., 333, 489-494 (1991).
- J. McManus, D. Cunningham, and M. J. Hynes, "Nuclear magnetic resonance and structural investigations of the chemistry of organotin compounds. II. 119Sn NMR investigations of the pyrazine adducts of dialkyltin(IV) dihalides", J. Organometal. Chem., 468, 87-92 (1994).
- B. Wrackmeyer, M. Vosteen, and W. Storch, "Precise measurements of 115/117/119Sn NMR frequencies and first observation of isotope-induced chemical shifts 1Δ 117/119Sn(15N), 1Δ 115/119Sn(15N), and 2Δ 117/119Sn(29Si) in N-trimethylstannyl amines", J. Mol. Struct., 602-603, 177-184 (2002).