(Xe) Xenon NMR
Use our NMR service that provides Xe NMR and many other NMR techniques.
Xenon has two medium to low sensitivity nuclei that have a very wide chemical shift range. 129Xe is a medium sensitivity spin ½ nucleus that yields sharp signals. 131Xe is a low sensitivity spin 3/2 nucleus that yields narrow lines in highly symmetrical environments and broader signals in less symmetrical environments (fig. 1). 129Xe is therefore the preferred nucleus although the quadrupolar relaxation of 131Xe is sometimes used as a probe for environmental asymmetry. Xenon NMR is used extensively as an inert physical probe of the solution and gas state in addition to studies of its chemistry and weak molecular bonding.
Fig. 1. Comparison of 129Xe and 131Xe spectra of xenon gas (23 atm) in CD3OD
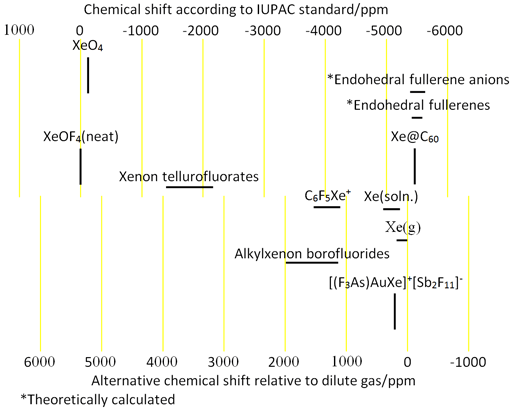
Having such a wide, chemical shift range (fig. 2), xenon's chemical shift is very susceptible to physical conditions. For example the chemical shift of xenon gas varies with pressure by 0.9 ppm/atm. The dissolved gas has a chemical shift range of approximately 200 ppm depending on the properties of the liquid. It is therefore an excellent indicator of the physical properties of the solution. Each type of xenon compound has its characteristic chemical shift range. The chemical shift scale is referenced by IUPAC to XeOF4 but is more usually referenced to low pressure xenon gas, a difference of 5386 ppm.
Fig. 2. Chemical shift ranges for xenon NMR
129Xenon NMR
129Xe is a medium sensitivity spin ½ nucleus that yields sharp signals (fig. 3). 129Xe is the xenon nucleus of choice because it is more sensitive and yields sharper signals than 131Xe.
Fig. 3. 129Xe spectrum of xenon gas (23 atm) in CD3OD
129Xe in molecules often couplings to other nuclei. Two and three bond couplings to fluorine are between 20 and 200 Hz. Couplings have also been reported to 1H, 13C, 14N, 15N, 17O and 125Te.
Properties of 129Xe
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 26.44% |
| Chemical shift range IUPAC | 5800 ppm, from -5700 to 100 |
| Chemical shift range rel. to gas | 5800 ppm, from -400 to 5400 |
| Frequency ratio IUPAC (Ξ) | 27.810186% |
| Frequency ratio rel. to gas (Ξ) | 27.6603909% |
| Reference compound | XeOF4 (neat) or Xe gas at low pressure |
| Linewidth of reference | 0.9 Hz* |
| T1 of reference | >200 s |
| Receptivity rel. to 1H at natural abundance | 5.72 × 10-3 |
| Receptivity rel. to 1H when enriched | 0.0216 |
| Receptivity rel. to 13C at natural abundance | 33.6 |
| Receptivity rel. to 13C when enriched | 127 |
*Theoretically the line should be much narrower but thermal inhomogeneity broadens the line.
131Xenon NMR
131Xe is a low sensitivity quadrupolar nucleus, less sensitive than 129Xe. Although it yields sharp signals in the gas phase it is broad even as dissovled gas (fig. 4) and its signals get much broader in asymmetric environments. 131Xe is therefore not the xenon nucleus of choice. However, the quadrupolar broadening of 131Xe is sometimes used as a measure of asymmetry in the molecular environment.
Fig. 4. 131Xe spectrum of xenon gas (23 atm) in CD3OD
Properties of 131Xe
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 21.18% |
| Chemical shift range IUPAC | 5800 ppm, from -5700 to 100 |
| Chemical shift range rel. to gas | 5800 ppm, from -400 to 5400 |
| Frequency ratio IUPAC (Ξ) | 8.243921% |
| Frequency ratio rel. to gas (Ξ) | 8.1995134% |
| Reference compound | XeOF4 (neat) or Xe gas at low pressure |
| Linewidth of reference | 0.7 Hz |
| T1 of reference | 1.4 s |
| Receptivity rel. to 1H at natural abundance | 5.96 × 10-4 |
| Receptivity rel. to 1H when enriched | 2.81 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 3.5 |
| Receptivity rel. to 13C when enriched | 16.5 |
| Linewidth parameter | 170 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, xenon displaces oxygen in the air and is therefore an asphyxiant. XeOF4 is unstable and reacts with water to yield HF that is highly corrosive, dissolves glass, very toxic, causes serious burns and other nasty biological damage including death, requires special equipment (chemical hood, gloves, eye protection, 2.5% calcium gluconate gel to treat burns by application and/or injection, etc.) and special skills to handle.
References
- F. J. Adrian, "Temperature and density dependence of the nuclear magnetic resonance chemical shift in xenon gas", Chem. Phys. Lett., 7, 201-204 (1970).
- C. J. Jameson amd A. K. Jameson, "Density dependence of xenon-129 NMR chemical shifts in oxygen and nitric oxide", Mol. Phys., 20, 957-959 (1971).
- R. A. Kromhout and B. Linder, "Xenon-129 solvent shift in various gases", J. Chem. Phys., 54, 1834-1835 (1971).
- K. Seppelt and H. H. Rupp, "Xenon-129 NMR spectra of xenon compounds. I. Simple xenon derivatives", Z. Anorg. Allgem. Chem., 409, 331-337 (1974).
- K. Seppelt and H. H. Rupp, "Xenon-129 NMR spectra of xenon compounds. II. Xenon(II) compounds", Z. Anorg. Allgem. Chem., 409, 338-342 (1974).
- C. J. Jameson, A. K. Jameson and S. M. Cohen, "Xenon-129 contact shift in oxygen gas", Mol. Phys., 29, 1919-1927 (1975).
- G. J. Schrobilgen, J. H. Holloway, P. Granger and C. Brevard, "Xenon-129 pulse Fourier-transform nuclear magnetic resonance spectroscopy", Inorg. Chem., 17, 980-987 (1978).
- K. W. Miller, N. V. Reo, U. Schoot, J. M Antonius D. P. Stengle, T. R. Stengle and K. L. Williamson, "Xenon NMR: chemical shifts of a general anesthetic in common solvents, proteins, and membranes", Proc. Nat. Acad. Sci., 78, 4946-4949 (1981).
- N. Muller, "Medium effects on fluorine-19 and xenon-129 chemical shifts in hydrocarbon solvents", J. Phys. Chem., 86, 2109-2110 (1982).
- N. V. Reo, "A study of nonspecific solute-solvent interactions in liquid solutions by xenon nuclear magnetic resonance spectroscopy - chemical shifts and spin-lattice relaxation" 144 pp. (1983).
- M. Claessens, O. Fabre, D. Zimmermann and J. Reisse, "NMR study of molecular interactions between xenon and crown ethers", Bull. Soc. Chim. Belg., 93, 983-989 (1984).
- T. R. Stengle, S. M. Hosseini and K. L. Williamson, "NMR chemical shifts of xenon in mixed aprotic solvents: A probe of liquid structure", J. Sol. Chem., 15, 777-790 (1986).
- J. Reisse, "Monoatomic xenon: its great interest in chemistry as a probe of intermolecular interactions, and its (easy) study by NMR", Nouv. J. Chim., 10, 665-672 (1986).
- A. Moschos and J. Reisse, "Nuclear magnetic relaxation of xenon-129 dissolved in organic solvents.", J. Magn. Res., 95, 603-606 (1991).
- E. M. Arnett and P. C. Wernett, "Does the basicity of xenon-129 affect its NMR chemical shift?", J. Am. Chem. Soc., 115, 12187-12188 (1993).
- C. I. Ratcliffe, "Xenon NMR", Ann. Rep. NMR Spectrosc., 36, 123-221 (1998).
- E. Locci, Y. Dehouck, M. Casu, G. Saba, A. Lai, M. Luhmer, J. Reisse and K. Bartik, "Probing proteins in solution by 129Xe NMR spectroscopy", J. Magn. Res., 150, 167-174 (2001).
- S. M. Rubin, M. M. Spence, I. E. Dimitrov, E. J. Ruiz, A. Pines and D. E. Wemmer, "Detection of a Conformational Change in Maltose Binding Protein by 129Xe NMR Spectroscopy", J. Am. Chem. Soc., 123, 8616-8617 (2001).
- M. S. Syamala, R. J. Cross and M. Saunders, "129Xe NMR of xenon inside C60", Proc. Electrochem. Soc., 2002-12(Fullerenes--Volume 12: The Exciting World of Nanocages and Nanotubes), 376-380 (2002).
- D. Raftery, "Xenon NMR spectroscopy", Ann. Rep. NMR Spectrosc., 57, 205-270 (2006).