(Mo) Molybdenum NMR
Use our NMR service that provides Mo NMR and many other NMR techniques.
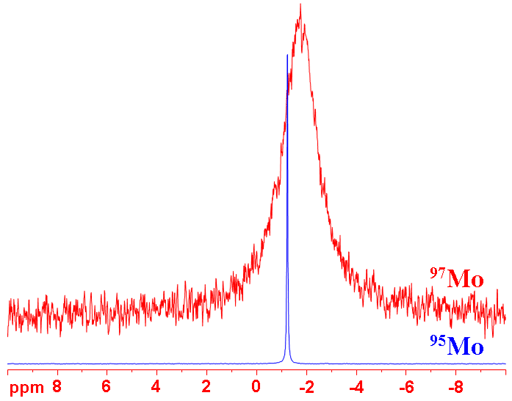
Molybdenum (Mo) has two NMR active nuclei that have a very wide chemical shift range. 95Mo and 97Mo, are both quadrupolar although 95Mo yields very narrow lines in small complexes. 95Mo is preferred as it has the higher sensitivity and yields much narrower signals than 97Mo (fig. 1). Molybdenum NMR is used for the study of molybdenum complexes and, using its relaxation rate, its binding to larger molecules.
Fig. 1. Comparison of the molybdenum isotopes under comparable conditions for Na2MoO4 (1 M) in D2O
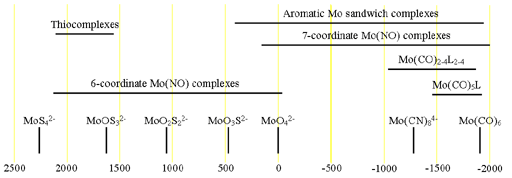
Both the isotopes have the same chemical shifts (fig. 2).
Fig. 2. Chemical shift ranges for molybdenum NMR
95Molybdenum NMR
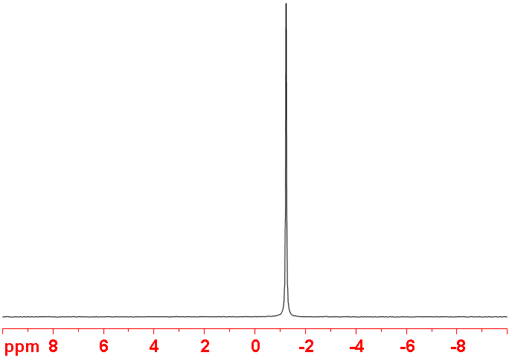
(95Mo) 95Molybdenum is a spin 5/2 nucleus and is therefore quadrupolar. As a result, the signal width increases with asymmetry of the environment but its very small quadrupole moment means that very sharp signals are observed (fig. 3) in small complexes containing one molybdenum atom. Larger complexes (2-4 molybdenums) yield lines that are hundreds to thousands Hertz wide. 95Mo is more sensitive and yields much sharper signals than 97Mo so 95Mo is the nucleus of choice for molybdenum NMR unless studying isotopic enrichment.
Fig. 3. 95Mo-NMR spectrum of Na2MoO4 (1 M) in D2O
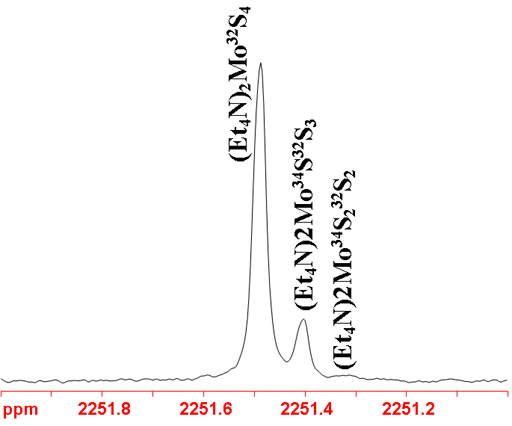
Because molybdenum has such a wide chemical shift range and 95Mo gives narrow signals, the slightest effect can be resolved as in the spectrum in fig. 4 where replacing 32S with 34S gives extra signals.
Fig. 4. 95Mo-NMR spectrum of of (Et4N)2MoS4 in D2O
Properties of 95Mo
| Property | Value |
|---|---|
| Spin | 5/2 |
| Natural abundance | 15.92% |
| Chemical shift range | 4300 ppm, from -2000 to 2300 |
| Frequency ratio (Ξ) | 6.516926% |
| Reference compound | 2 M Na2MoO4 in D2O |
| Linewidth of reference | 0.52 Hz |
| T1 of reference | 0.81 s |
| Receptivity rel. to 1H at natural abundance | 5.21 × 10-4 |
| Receptivity rel. to 1H when enriched | 3.27 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 3.06 |
| Receptivity rel. to 13C when enriched | 19.2 |
| Linewidth parameter | 1.5 fm4 |
97Molybdenum NMR
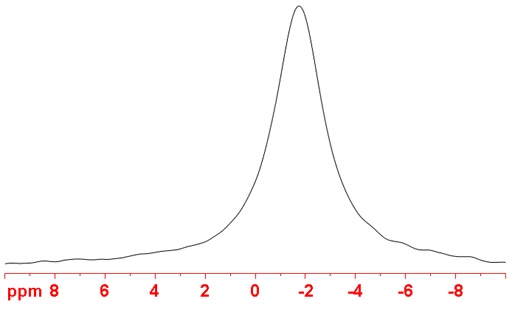
(97Mo) 97Molybdenum is a spin 5/2 nucleus and is therefore quadrupolar. As a result, the signal width increases with asymmetry of the environment. 97Mo is less sensitive and yields much wider lines (fig. 5) than 95Mo so is not the molybdenum nucleus of choice.
Fig. 5. 97Mo-NMR spectrum of Na2MoO4 (1 M) in D2O
Properties of 97mo
| Property | Value |
|---|---|
| Spin | 5/2 |
| Natural abundance | 9.55% |
| Chemical shift range | 4300 ppm, from -2000 to 2300 |
| Frequency ratio (Ξ) | 6.653695% |
| Reference compound | 2 M Na2MoO4 in D2O |
| Linewidth of reference | 42 Hz |
| T1 of reference | 0.007 s |
| Receptivity rel. to 1H at natural abundance | 3.28 × 10-4 |
| Receptivity rel. to 1H when enriched | 3.43 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 1.96 |
| Receptivity rel. to 13C when enriched | 20.5 |
| Linewidth parameter | 210 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, molybdenum salts may be toxic.
References
- O. Lutz, A. Nolle and P. Kroneck, "Use of 95Mo NMR for identification of molybdenum (VI) chalcogenide anions in aqueous solution", Z. Naturforsch. A, 32, 505-506 (1977).
- A. F. Masters, R. T. C. Brownlee, M. J. O'Connor, A. G. Wedd and J. D. Cotton, "Molybdenum-95 nuclear magnetic resonance. Applications to substituted carbonyls", J. Organometal. Chem., 195, C17-C20 (1980).
- P. Kronech, O. Lutz and A. Nolle, "NMR investigations of 17O, 33S, 95Mo, and 97Mo in thiomolybdates", Z. Naturforsch. A, 35, 226-229 (1980).
- S. Dysart, I. Georgii and B. E. Mann, "Molybdenum-95 NMR spectra of some molybdenum carbonyl and related compounds", J. Organometal. Chem., 213, C10-C12 (1981).
- G. M. Gray and R. J. Gray, "Synthesis and a multinuclear spectroscopic study of some Mo(CO)2(PPh2XR) (X = O, NH; R = 1-4 carbon alkyls) complexes. Steric effects on 31P and 95Mo chemical shifts", Organometal., 2, 1026-1031 (1983).
- S. F. Gheller, T. W. Hambley. R. T. C. Brownlee, M. J. O'Connor , M. R. Snow and A. G. Wedd, "Applications of molybdenum-95 NMR spectroscopy. 7. Studies of metal-metal bonded systems including aqueous molybdenum(IV) and molybdenum(V). Crystal and molecular structure of Na2[Mo3O4((O2CCH2)2NCH3)3].7H2O", J. Am. Chem. Soc., 105, 1527-1532 (1983).
- J. W. Faller and B. C. Whitmore, "Analysis of conformation isomers of molybdenum allyl complexes using 95Mo NMR spectroscopy", Organometal., 5, 752-755 (1986).
- J. C. Green, R. A. Grieves and J. Mason, "Molybdenum-95 and carbon-13 nuclear magnetic shielding and bonding relationships in sandwich complexes", J. Chem. Soc. Dalton Trans., 1313-1316 (1986).
- E. C. Alyea, A. Malek and J. Malito, "95Mo NMR studies of some carbonylate anions", Polyhedron, 5, 403-406 (1986).
- C. G. Young, M. Minelli, J. H. Enemark, W. Hussain, C. J. Jones and J. A. McCleverty, "A molybdenum-95 and nitrogen-14 nuclear magnetic resonance study of six-co-ordinate hydrotris(3,5-dimethyl-1-pyrazoyl)borate complexes containing a sixteen-electron {Mo(NO)}4 core", J. Chem. Soc. Dalton Trans., 619-621 (1987).
- E. C. Alyea and A. Somogyvari, "Molybdenum-95 nuclear magnetic resonance studies on disubstituted molybdenum(0) carbonyls", Can. J. Chem., 66, 397-400 (1988).
- H, Schumann, J. H. Enemark, M. J. Labarre, M. Bruck and P. Wexler, "95Mo NMR investigation on cationic [C5H5Mo(CO)2L2]BF4 complexes (L = group 15 donor ligands)", Polyhedron, 10, 665-671 (1991).
- T. C. Wong, Q. Huang, N. Zhu and X. Wu, "95Mo NMR studies of five heterometallic trinuclear incomplete cubane-like clusters", Polyhedron, 16, 2987-2990 (1997).