Quadrupolar nuclei
A quadrupolar nucleus is one that has a quantum spin number greater than ½. Such nuclei have a lower symmetry than spin-½ nuclei. The quadrupole moment that varies between nuclei is a measure of this asymmetry.
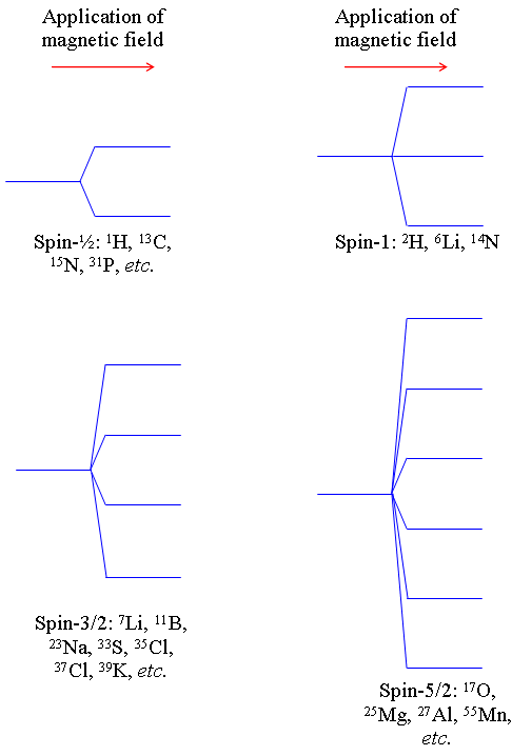
Their energies split upon the application of a magnetic field into multiple levels (fig. 1). By comparison, spin-½ nuclei split into only two levels. The number of levels is given by 2n + 1 where is the spin number. The different splitting patterns can be seen in their coupling with other nuclei.
Fig. 1. Energy level diagrams for nuclei of different spins
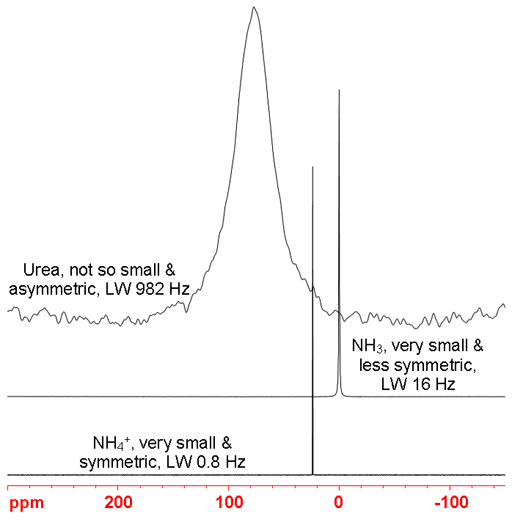
The NMR signals of quadrupolar nuclear are usually wider than those of spin-½ nuclei due to rapid quadrupolar relaxation. The line-width increases with the line-width factor that is related to the quadrupole moment of the nucleus, the size and asymmetry of the molecule. For example, deuterium has a small line-width factor of 0.41 fm4 with a quadrupole moment of 0.286 fm2 and yields a line-width in HOD of 1.7 Hz while 17O has a line-width factor of 2.1 fm4 with a quadrupole moment of -2.56 fm2 and yields a line-width in H217O of 69 Hz. Some nuclei such as 197Au and 201Hg yield signals too broad to be observed with a high-resolution NMR spectrometer. The effect of shape and size of the molecule can be demonstrated with 14N-NMR (fig. 2). NH4+ is small and highly symmetric yielding a line-width of 0.8 Hz, NH3 is also small but asymmetric giving a line-width of 16 Hz. Urea is larger and even less symmetric yielding a line-width of 982 Hz.
Fig. 2. Comparison of line-widths of 14N signals. The larger and less symmetric the molecule, the wider the signal.
In solid-state NMR, the methods applied to quadrupolar nuclei differ from those applied to spin-½ nuclei. There is also a difference between whole integer and half integer nuclei. Whole integer nuclei yield very broad spectra while half integer nuclei yield a relatively narrow central transition in addition to a very broad multiple quantum signal. The central transition can be used to yield high-resolution information from the spectrum.