2D 13C-13C INADEQUATE
2D 13C-13C INADEQUATE (Incredible Natural Abundance DoublE QUAntum Transfer Experiment) is useful for determining which signals arise from neighboring carbons. However, it is very insensitive as 0.01% of the carbons are excited at natural abundance. Use this experiment as a last resort when all else fails. If there are protons within six bonds of the carbons of interest, consider using 2D-ADEQUATE instead. If you can enrich the sample to about 10% 13C then the INADEQUATE experiment can be run with normal sample concentrations. At natural abundance, it is recommended to use between 100 and 500 mg of sample. If it will not completely dissolve in 0.8 mL of solvent then use a 10 mm NMR tube and 2.5 mL of solvent. A 2D INADEQUATE spectrum yields one bond correlations via spin-spin coupling. The spectrum does not appear in the same form as a COSY spectrum but rather the f2 axis is the carbon chemical shift and the f1 axis is the double quantum frequency, i.e. the sum of the two correlating frequencies. There are no diagonal signals. Signals with similar chemical shifts loose sensitivity due to second order coupling.
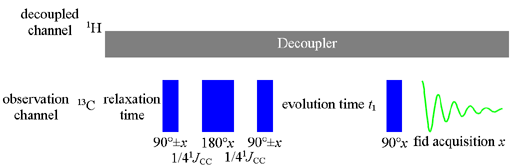
In the 2D INADEQUATE pulse sequence (fig. 1) DELTA should be set to 1/(4J) where J is the one-bond CC coupling. The one-bond coupling constants can be used to estimate carbon-carbon bond order. The constants are usually 35 to 45 for a single bond and about 65 Hz for a double bond and are increased by electronegative substituents. If the only purpose is to measure coupling constants then 1D-INADEQUATE is a more suitable experiment.
Fig. 1. Pulse sequence for 2D INADEQUATE
The spectrum is laid out differently than other homonuclear 2D spectra. The cross-peaks are distributed symmetrically and horizontally either side of the diagonal (red dashed line in fig. 2). The connection between each pair of carbons is shown as a solid red line (fig. 2). Each cross-peak is a doublet split by carbon-carbon coupling. In the 2D-INADEQUATE spectrum (fig. 2) of ethylbenzene (fig.3), the carbon connectivity can be followed through the molecule from 2' to 1' to 1 to 2 to 3 to 4.
Fig. 2. 2D INADEQUATE spectrum of ethylbenzene in C6D6
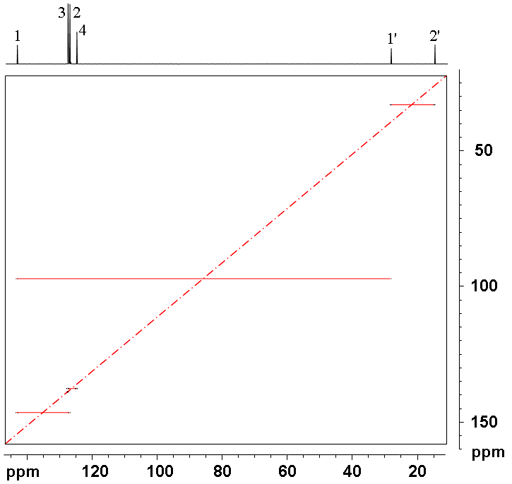
Fig. 3. Structure of ethylbenzene
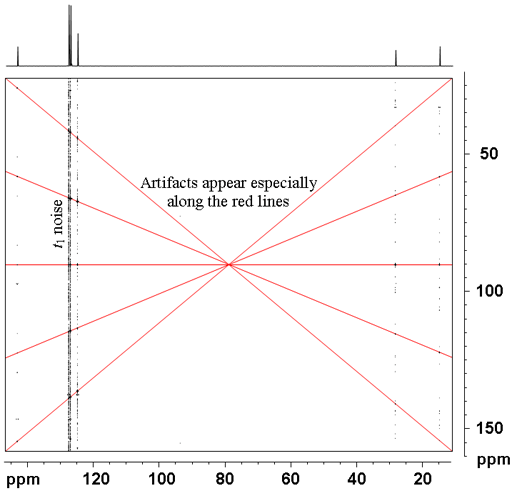
However, 2D-INADEQUATE is very prone to artifacts (fig. 4), especially vertical streaks of t1 noise.
Fig. 4. 2D INADEQUATE spectrum of ethylbenzene showing spectral artifacts
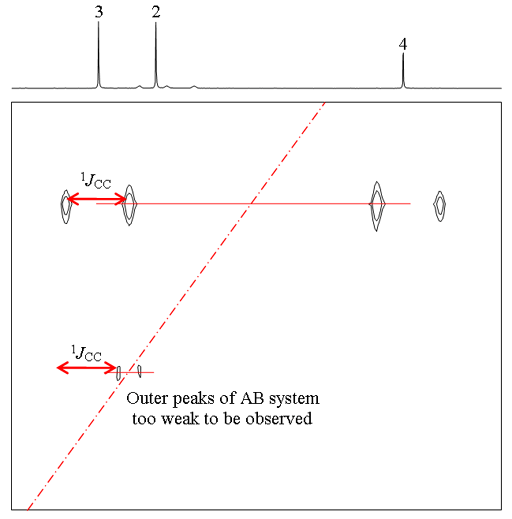
When two neighboring carbons have similar chemical shifts, the cross-peak is weaker and the doublets form a second order AB coupling pattern as shown below for the correlation between C2 and 3 of ethylbenzene (fig. 5).
Fig. 5. 2D INADEQUATE spectrum of ethylbenzene showing second order coupling