(Os) Osmium NMR
Use our NMR service that provides 189Os NMR and many other NMR techniques.
Osmium (Os) has two NMR active nuclei 187Os and 189Os. 189Os is a quadrupolar low sensitivity nucleus that yields a very broad signal for the highly symmetrical OsO4 molecule and cannot be observed for other molecules using a high resolution NMR spectrometer. 187Os is a spin ½ nucleus and yields sharp signals over a very wide chemical shift range. However, 187Os has an extremely low sensitivity so is usually observed in complexes by indirect detection via proton coupling. Osmium NMR is used for studying osmium complexes.
Each type of osmium has its characteristic chemical shift range (fig. 1).
Fig. 1. Chemical shift ranges for osmium NMR
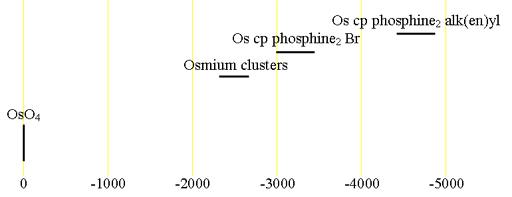
187Osmium NMR
187Os yields sharp signals however, it is much less sensitive and than 189Os. Therefore 187Os is so insensitive that it is usually studied by indirect detection via proton coupling. It is still the nucleus of choice because only OsO4 can be studied using 189Os NMR.
One-bond 1H-187Os couplings are between 25 and 85 Hz, and two-bond are about 5 Hz. One-bond 13C-187Os couplings are about 100 Hz and 31P-187Os couplings are about 300 Hz.
We have not succeeded in observing 187Os NMR in our laboratory due to its low sensitivity although its observation is possible and we are willing to make the extra effort to do so if the need arises. We do not have the equipment observe 187Os by indirect detection.
Properties of 187Os
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 1.96% |
| Chemical shift range | 5000 ppm, from -5000 to 0 |
| Frequency ratio (Ξ) | 2.282331% |
| Reference compound | 0.98 M OsO4 in CCl4 |
| Linewidth of reference | 0.1 Hz |
| T1 of reference | ~10 s |
| Receptivity rel. to 1H at natural abundance | 2.43 × 10-7 |
| Receptivity rel. to 1H when enriched | 1.24 × 10-5 |
| Receptivity rel. to 13C at natural abundance | 1.43 × 10-3 |
| Receptivity rel. to 13C when enriched | 0.073 |
189Osmium NMR
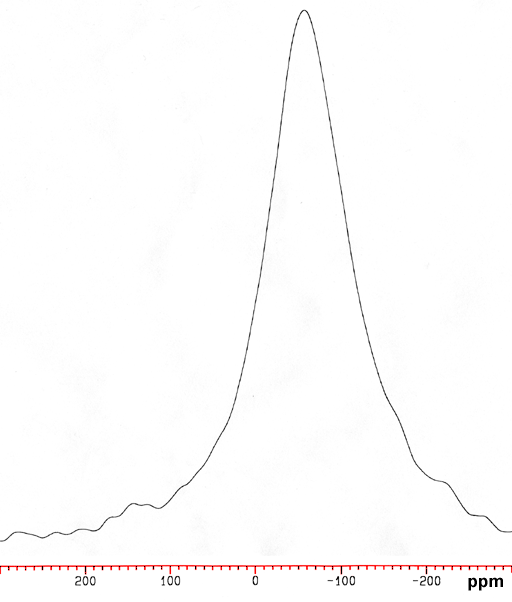
189Os is a quadrupolar nucleus that yields very broad signals for the most symmetrical of compounds but is much more sensitive and than 187Os. However, 189Os signals are too broad cannot be observed on a high resolution NMR spectrometer with the exception of OsO4. Therefore, with the exception of OsO4, 189Os is not the osmium nucleus of choice for NMR. Quadrupolar signals increase in line-width with increasing magnetic field. In our experience, OsO4 yields a broad signal on a 200 MHz spectrometer (fig. 2) and is unobservable on a 400 MHz spectrometer.
Fig. 2. 189Os NMR for OSO4 (1 M) in CCl4
Properties of 189Os
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 16.15% |
| Chemical shift range | 5000 ppm, from -5000 to 0 |
| Frequency ratio (Ξ) | 7.765400% |
| Reference compound | 0.98 M OsO4 in CCl4 |
| Linewidth of reference | 1800 Hz |
| T1 of reference | 0.0004 s |
| Receptivity rel. to 1H at natural abundance | 3.95 × 10-4 |
| Receptivity rel. to 1H when enriched | 2.02 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 2.32 |
| Receptivity rel. to 13C when enriched | 14.4 |
| Linewidth parameter | 9800 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, osmium salts are toxic and osmium tetroxide causes burns: wear eye protection and protective gloves.
References
- B. E. Mann, C. Masters and B. L. Shaw, "1J(osmium-187-hydrogen) coupling in the proton nuclear magnetic resonance spectra of some osmium hydrides" J. Chem. Soc. D: Chem. Comm., 1041 (1970).
- J. A. Cabeza, B. E. Mann, C. Brevard and P. M. Maitlis, "Dinuclear p-cymeneosmium hydride complexes: the measurement of osmium-187 chemical shifts using 1H-187Os two-dimensional NMR spectroscopy", J. Chem. Soc., Chem. Comm., 65-67 (1985).
- R. Benn, E. Joussen, H. Lehmkuhl, F. Lopez Ortiz and A. Rufinska, "Determination of d(187Os), J(Os,X), and T1(187Os) in osmium complexes via indirect 2D NMR spectroscopy" J. Am. Chem. Soc., 111, 8754-8756 (1989).