(Ti) Titanium NMR
Use our NMR service that provides Ti NMR and many other NMR techniques.
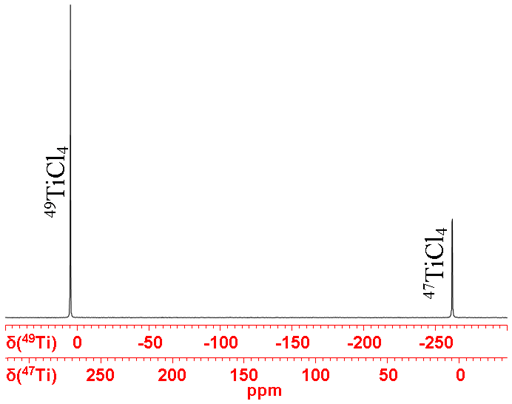
Titanium (Ti) has two low sensitivity NMR active nuclei, 47Ti and 49Ti, with a very wide chemical shift range. It is unique in that their resonant frequency difference is only 267 ppm so both nuclei are generally observed at the same time (fig. 1).
Fig. 1. Ti-NMR spectrum of TiCl4(neat) showing both nuclei in the same spectrum
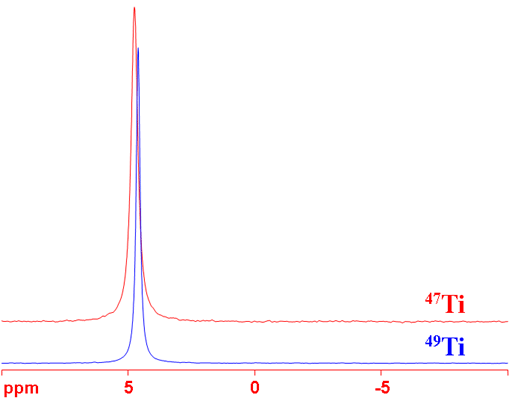
49Ti is the nucleus of choice because it is more sensitive and yields narrower lines than 47Ti (fig. 2). Both nuclei yield moderately sharp lines in symmetric environments that become wider with increasing size of the complex and too broad to observe with a high-resolution NMR spectrometer for medium sized complexes. Ti-NMR is therefore restricted to the study of small complexes.
Fig. 2. Comparison of 47Ti and 49Ti for TiCl4 (neat). 49Ti yields greater sensitivity and narrower signals.
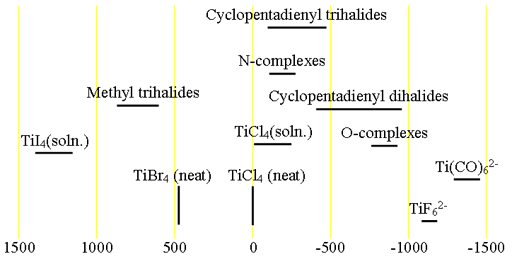
Each type of titanium compound has its characteristic chemical shift range (fig. 3). The NMR of Titanium can be observed in its (-II), (0), (II) and (IV) oxidation states. However, Ti(III) is paramagnetic and cannot be observed in high resolution NMR. Heteronuclear titanium spin-spin couplings have been observed with fluorine in TiF62- (33 Hz) and with 13C in Ti(13CO)62- (23 Hz).
Fig. 3. Chemical shift ranges for titanium NMR
47Titanium NMR
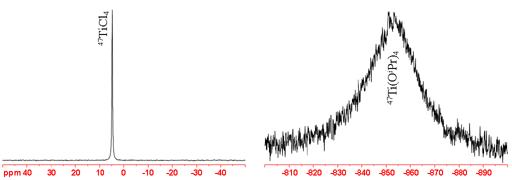
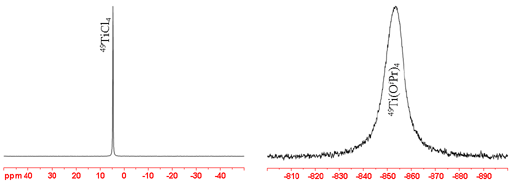
47Ti not as good an NMR nucleus as 49Ti is because it is less sensitive and yields broader signals. However, its signals appear about 267 ppm downfield of those of 49Ti unless they are too broad to observe. 47Ti yields moderately sharp lines in small symmetric molecules but its signals become rapidly wider (fig. 4) (more rapidly than for 49Ti) with increasing size of the complex and too broad to observe with a high-resolution NMR spectrometer for medium sized complexes. 47Ti-NMR can only be observed in the smallest of complexes.
Fig. 4. Comparison of 47Ti-NMR spectra of neat TiCl4 and Ti(OiPr)4 showing increasing line-width with molecular size
Properties of 47Ti
| Property | Value |
|---|---|
| Spin | 5/2 |
| Natural abundance | 7.44% |
| Chemical shift range | 2800 ppm, from -1400 to 1400 |
| Frequency ratio (Ξ) | 5.637534% |
| Reference compound | TiCl4 (90%) in C6D12* |
| Linewidth of reference | 5.2 Hz |
| T1 of reference | 0.06 s |
| Receptivity rel. to 1H at natural abundance | 1.56 × 10-4 |
| Receptivity rel. to 1H when enriched | 2.10 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 0.918 |
| Receptivity rel. to 13C when enriched | 12.3 |
| Linewidth parameter | 290 fm4 |
*Note that pure TiCl4 resonates at 4.8 ppm in the 47Ti-NMR spectrum.
49Titanium NMR
49Ti preferred as an NMR nucleus over 47Ti because it is more sensitive and yields shaper signals. 49Ti yields moderately sharp lines in small symmetric molecules but its signals become wider with increasing size of the complex (fig. 5) and too broad to observe with a high-resolution NMR spectrometer for medium sized complexes. 49Ti-NMR can only be observed in small complexes.
Fig. 5. Comparison of 49Ti-NMR spectra of neat TiCl4 and Ti(OiPr)4 showing increasing line-width with molecular size
Properties of 49Ti
| Property | Value |
|---|---|
| Spin | 7/2 |
| Natural abundance | 5.41% |
| Chemical shift range | 2800 ppm, from -1400 to 1400 |
| Frequency ratio (Ξ) | 5.639037% |
| Reference compound | TiCl4 (90%) in C6D12* |
| Linewidth of reference | 2.4 Hz |
| T1 of reference | 0.2 s |
| Receptivity rel. to 1H at natural abundance | 2.05 × 10-4 |
| Receptivity rel. to 1H when enriched | 3.79 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 1.20 |
| Receptivity rel. to 13C when enriched | 22.2 |
| Linewidth parameter | 83 fm4 |
*Note that pure TiCl4 resonates at 4.6 ppm in the 49Ti-NMR spectrum.
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, TiCl4 reacts violently with moist air releasing toxic fumes (HCl) and many other Ti(IV) compounds are unstable and poisonous: wear protective gloves and work in a hood under nitrogen.
References
- R. G. Kidd, R. W. Matthews and H. P. Spinney, "Titanium-47 and titanium-49 nuclear magnetic resonance in titanium(IV)-halogen compounds" J. Am. Chem. Soc., 94, 6686 (1972).
- N. Hao, B. G. Sayer, G. Dénès, D. G. Bickley, C. Detellier and M. J. McGlinchey, "Titanium-47 and -49 Nuclear Magnetic Resonance Spectroscopy: Chemical Applications" J. Magn. reson., 50, 50-63 (1982).
- A. Dormond, M. Fauconet, J. C. Leblanc and C. Moise, "First NMR observation of 47Ti and 49Ti of cyclopentadienyl complexes" Polyhdron, 3, 897-900 (1984).
- K. M. Chi, S. R. Frerichs, S. B. Philson and J. E. Ellis, "Highly Reduced Organometallic. 23. Synthesis, Isolation, and Characterization of Hexacrbonyltitanate(2-), Ti(CO)62-. Titanium NMR Spectra of Carbonyltitanates" J. Am. Chem. Soc., 110, 304-304 (1988).
- W. C. Finch, E. V. Anslyn and R. H. Grubbs, "Substituent Effects on the Cleavage Rates of Titanocene Metallacyclobutanes" J. Am. Chem. Soc., 110, 2406-2413 (1988).
- S. Berger , W. Bock, C. F. Marth, B. Raguse and M. T. Reetz, "47/49Ti NMR of some titanium compounds" Magn. Reson. Chem., 28, 559-560 (1990).