(Li) Lithium NMR
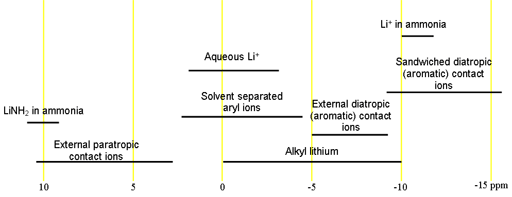
Lithium has two useful nuclei. 6Li has a spin of one. Spins of greater than ½ are quadrupolar but 6Li has a low quadrupolar moment and yields sharp signals but has low sensitivity. On the other hand, 7Li is highly sensitive but has a higher quadrupolar moment so its signals are broader. Both nuclei have a moderate chemical shift range. Each type of signal has a characteristic chemical shift range (fig. 1) which is the same for both nuclei.
Fig. 1. Chemical shift ranges for lithium NMR
6Lithium
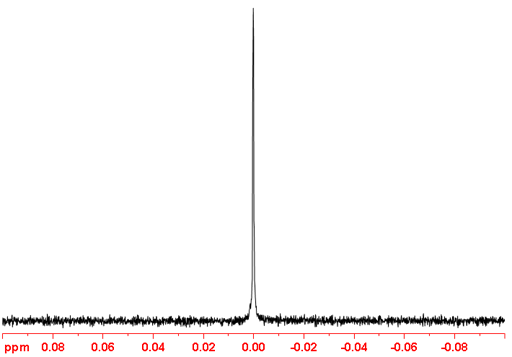
6Li-NMR (fig. 2) is less sensitive than 7Li even when enriched.
Fig. 2. 6Li-NMR spectrum of LiCl (1M) in D2O at natural abundance
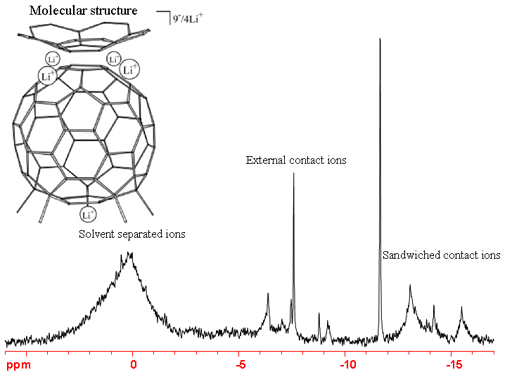
However, 6Li yields much shaper lines than 7Li, especially in asymmetric environments. As a result, it is possible to observe two-bond 1H-6Li and 13C-6Li couplings of the order of 1 Hz in covalently bonded molecules. 6Li tends to have long T1 relaxation times so a preacquisition delay of 5 to 10 seconds is usually required. The spectrum (fig. 3) shows a large number of signals arising from a supramolecular complex. Some signals are very sharply defined with 1 Hz line-width. However, sensitivity is limited even though the sample was enriched with 6Li.
Fig. 3. 6Li-NMR spectrum of Me5C605-/Cor4-/9Li+ showing signals in three separate regions
(see I. Aprahamian, et al., J. Am. Chem. Soc., 127, 9581-7 (2005))
Properties of 6Li
| Property | Value |
|---|---|
| Spin | 1 |
| Natural abundance | 7.59% |
| Chemical shift range | 28 ppm, from -16 to 11 |
| Frequency ratio (Ξ) | 14.716086% |
| Reference compound | 9.7 m LiCl in D2O |
| Linewidth of reference | 0.03 Hz |
| T1 of reference | ~150 s |
| Receptivity rel. to 1H at natural abundance | 6.45 × 10-4 |
| Receptivity rel. to 1H when enriched | 8.50 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 3.79 |
| Receptivity rel. to 13C when enriched | 49.9 |
| Linewidth parameter | 0.033 fm4 |
7Lithium
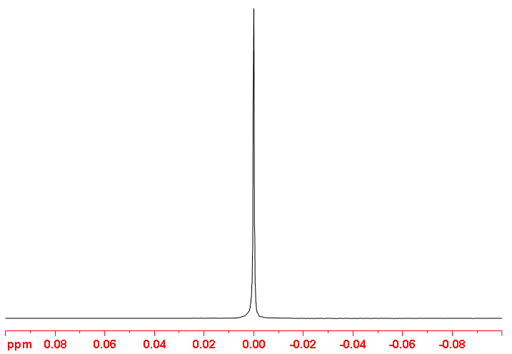
7Li-NMR is more sensitive than 6Li but yields broader signals due to its higher quadrupolar moment. The broadening is not very evident for aqueous lithium ions because they are very small and symmetrical (fig. 4).
Fig. 4. 7Li-NMR spectrum of LiCl (1M) in D2O
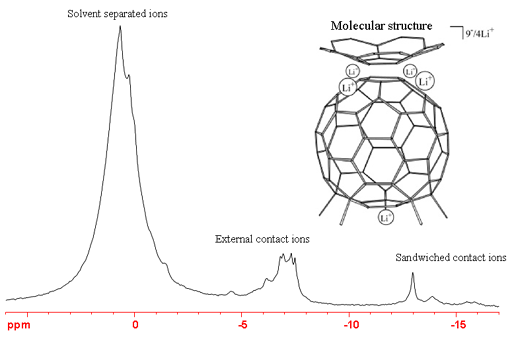
However, for organometallic systems with lithium bonded within a molecule, it has a short relaxation time, typically 0.2 s. In covalent and π-bonded systems this allows fast repetition times and high signal-to-noise. Coupling to 7Li is very rarely observable with very few cases of one-bond 1H-7Li coupling (about 20 Hz) known. The 7Li spectrum (fig. 5) is much more sensitive than the 6Li spectrum (fig. 3). Low symmetry combined with the higher quadrupole moment of 7Li broaden the signals. As a result, the signals that were tall and sharp in the 6Li spectrum (fig. 3) are now low and broad (fig. 5).
Fig. 5. 7Li-NMR spectrum of Me5C605-/Cor4-/9Li+ showing signals in three separate regions
(see I. Aprahamian, et al., J. Am. Chem. Soc., 127, 9581-7 (2005))
Properties of 7Li
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 92.41% |
| Chemical shift range | 28 ppm, from -16 to 11 |
| Frequency ratio (Ξ) | 38.863797% |
| Reference compound | 9.7 m LiCl in D2O |
| Linewidth of reference | 0.07 Hz |
| T1 of reference | 36 s |
| Receptivity rel. to 1H at natural abundance | 0.271 |
| Receptivity rel. to 1H when enriched | 0.29 |
| Receptivity rel. to 13C at natural abundance | 1590 |
| Receptivity rel. to 13C when enriched | 1721 |
| Linewidth parameter | 21 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Lithium salts are not toxic on account of the lithium under normal circumstances; however, the anion may be toxic.