(133Cs) Cesium NMR
Use our NMR service that provides 133Cs NMR and many other NMR techniques.
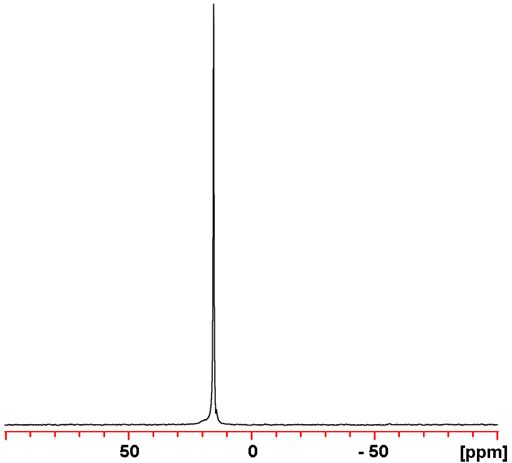
Cesium (Cs) has one medium sensitivity NMR active nucleus, 133Cs (fig. 1). It is quadrupolar but has a very low quadrupole moment and is usually found in symmetric environments and yields narrow signals over a wide chemical shift range. 133Cesium NMR is mostly used for studying cesium complexes and detecting binding.
Fig. 1. 133Cs-NMR spectrum of CsCl (1 M) in D2
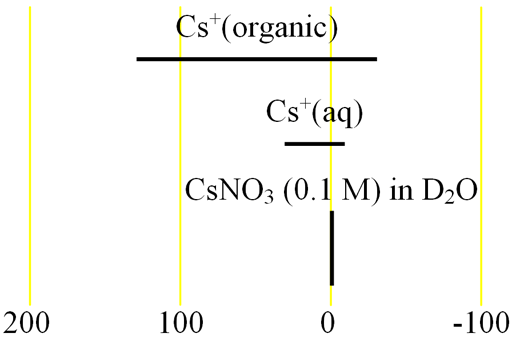
Each type of cesium its representative chemical shift range (fig. 2).
Fig. 2. Chemical shift ranges for cesium NMR
Properties of 133Cs
| Property | Value |
|---|---|
| Spin | 7/2 |
| Natural abundance | 100% |
| Chemical shift range | 160 ppm, from -30 to 130 |
| Frequency ratio (Ξ) | 13.116142% |
| Reference compound | 0.1 M CsNO3 in D2O |
| Linewidth of reference | 0.12 Hz |
| T1 of reference | 5 s |
| Receptivity rel. to 1H at natural abundance | 0.0484 |
| Receptivity rel. to 1H when enriched | 0.0484 |
| Receptivity rel. to 13C at natural abundance | 284 |
| Receptivity rel. to 13C when enriched | 284 |
| Linewidth parameter | 0.016 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, cesium salts are toxic in large quantities besides any toxicity arising from the anion.
References
- J. H. Halliday, H. D. W. Hill, R. E. Richards, "Solvent isotope shifts of cesium resonances in dilute salt solutions", Chem. Commun., 219-220 (1969).
- E. Mei, L. Liu, J. L. Dye and A. I. Popov, "Determination of stability constants of cesium[2]-cryptand complexes in nonaqueous solvents by cesium-133 NMR", J. Soln. Chem., 6, 771-778 (1977).
- H. Gustavsson, T. Ericsson and B. Lindman, "133Cs NMR chemical shifts in mixtures of NN-dimethylformamide and water", Norg. Nucl. Chem. Lett., 14, 37-43 (1978).
- H. Gustavsson and B. Lindman, "Alkali ion binding to aggregates of amphiphilic compounds studied by nuclear magnetic resonance chemical shifts", J. Am. Chem. Soc., 100, 4647-4654 (1978).
- S. Khazaeli, A. I. Popov and J. L. Dye "Cesium-133 nuclear magnetic resonance study of the complexation of cesium salts by 18-crown-6 in methylamine. 1. 1:1 complex formation", J. Phys. Chem., 86, 5018-5023 (1982).
- G. Bonas and M. R. Vignon, "Cesium-133 nuclear magnetic resonance study of cyclogentiotriose peracetate-cesium picrate complexation in non-aqueos solutions", Carbohydr. Res., 192, 343-346 (1989).
- V. N. Mirny, V. V. Trachevski and T. A Mirnaya, "Nuclear magnetic resonance study of cesium-133 in binary molten trifluoroacetate salt mixtures", Z. Naturforsch. A, 56, 288-290 (2001).
- R. M. Wellard and W. R. Adam, "Functional hepatocyte cation compartmentation demonstrated with 133Cs NMR", Magn. Reson. Med., 48, 810-818 (2002).