(Ag) Silver NMR
Use our NMR service that provides Ag NMR and many other NMR techniques.
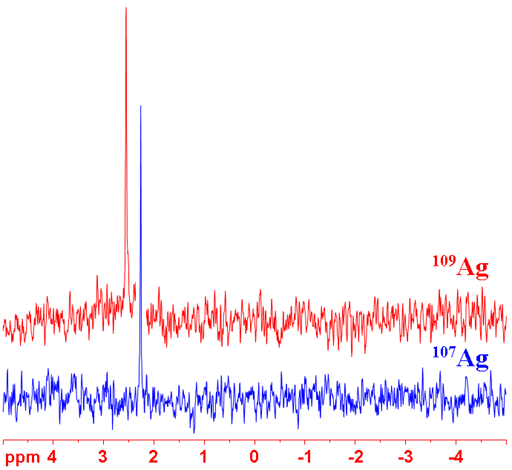
Silver (Ag) has two NMR active spin ½ nuclei that yield very narrow signals over a wide chemical shift range. 107Ag and 109Ag both have very low sensitivity, 109Ag being preferred as it has slightly higher sensitivity (fig. 1). In addition, 107Ag has a resonant frequency beyond that of most standard NMR probes. However, it may be that 107Ag yields sharper signals although it is difficult to determine for sure and resolution is not usually the major problem with silver NMR. Silver NMR is used for the study of silver salts and complexes. Its relaxation time is very long, of the order of hundreds of seconds for degassed samples and tens of seconds for air saturated samples, so the addition of a relaxation agent such as iron(III)nitrate may be used to speed acquisition.
Fig. 1. Comparison of the silver NMR isotopes for a sample of AgNO3 (1 M) in D2O
Both the isotopes have the same chemical shifts (fig. 2). The chemical shift is very sensitive to concentration and temperature.
Fig. 2. Chemical shift ranges for silver NMR

107Silver NMR
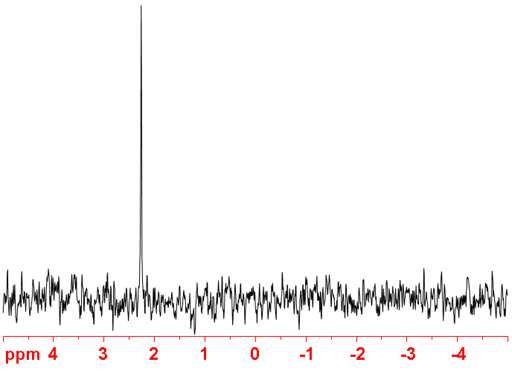
(107Ag) 107Silver is a spin ½ nucleus that yields very sharp signals but is very insensitive (fig. 3). It is slightly less sensitive than 109Ag so is not normally the preferred silver isotope for NMR. 107Ag has a resonant frequency beyond that of most standard NMR probes. However, there is some evidence, although not certain, that the 107Ag signal may be narrower than the 109Ag signal.
Fig. 3. 107Ag-NMR spectrum of AgNO3 (1 M) in D2O
Properties of 107Ag
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 51.839% |
| Chemical shift range | 750 ppm, from -25 to 725 |
| Frequency ratio (Ξ) | 4.047819% |
| Reference compound | AgNo3 (sat.) in D2O |
| Linewidth of reference | 0.15 Hz |
| T1 of reference | ~400 s |
| Receptivity rel. to 1H at natural abundance | 3.50 × 10-5 |
| Receptivity rel. to 1H when enriched | 6.75 × 10-5 |
| Receptivity rel. to 13C at natural abundance | 0.205 |
| Receptivity rel. to 13C when enriched | 0.395 |
109Silver NMR
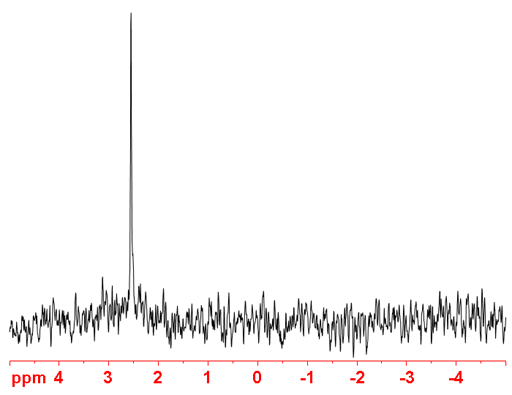
(109Ag) 109Silver is a spin ½ nucleus that yields very sharp signals but is very insensitive (fig. 4). It is slightly more sensitive that 107Ag and so is the preferred silver isotope for NMR.
Fig. 4. 109Ag-NMR spectra of AgNO3 (1 M) in D2O
Properties of 109Ag
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 48.161% |
| Chemical shift range | 750 ppm, from -25 to 725 |
| Frequency ratio (Ξ) | 4.653533% |
| Reference compound | AgNo3 (sat.) in D2O |
| Linewidth of reference | 0.2 Hz |
| T1 of reference | 420 s |
| Receptivity rel. to 1H at natural abundance | 4.94 × 10-5 |
| Receptivity rel. to 1H when enriched | 1.03 × 10-4 |
| Receptivity rel. to 13C at natural abundance | 0.290 |
| Receptivity rel. to 13C when enriched | 0.602 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, silver salts are toxic (LD50 for AgNO3 is 7 g): wear protective gloves.
References
- C. W. Burges, R. Koschmieder, W. Sahm, and A. Schwenk, "Silver-107 and silver-109 nuclear magnetic resonance studies of silver ions in aqueous solutions", Z. Naturforsc. A, 28, 1753-1758 (1973).
- P. M. Henrichs, S. Sheard, J. J. H Ackerman and G. E. Maciel, "Structural studies of organic silver complexes in dimethyl sulfoxide by carbon-13 and silver-109 NMR", J. Am. Chem. Soc., 101, 3222-3228 (1979).
- A. F. M. J. Van der Ploeg, G. Van Koten, C. Brevard, "INEPT silver-109 NMR evidence for direct platinum-to-silver bonding in dinuclear [2,6-(Me2NCH2)2C6H3][p-tolNC(H)NR]PtAgBr]", Inorg. Chem., 21, 2878-2881 (1982).
- K. Endo, K. Matsushita, K. Deguchi, K. Yamamoto, S. Suzuki and K. Futaki, "Silver-109 NMR spectra of aqueous silver ions coordinated with nitroxide radical. Silver-tanol complex and tanol as a doping agent", Chem. Lett., 1497-1500 (1982).