(Ga) Gallium NMR
Use our NMR service that provides Ga NMR and many other NMR techniques.
Gallium (Ga) has two NMR active nuclei, 69Ga and 71Ga (fig. 1) with a very wide chemical shift range (fig. 2). Both nuclei are quadrupolar and therefore yield broad signals in small molecules and signals, too broad to be observed with a high-resolution NMR spectrometer, in medium and large molecules. 71Ga is the more sensitive nucleus and yields narrower signals than 69Ga so 71Ga is the nucleus of choice unless studying isotopic enrichment.
Fig. 1. Comparison of 69Ga and 71Ga for Ga(NO3)3 in D2O. 71Ga is the more sensitive nucleus and yields narrower signals.
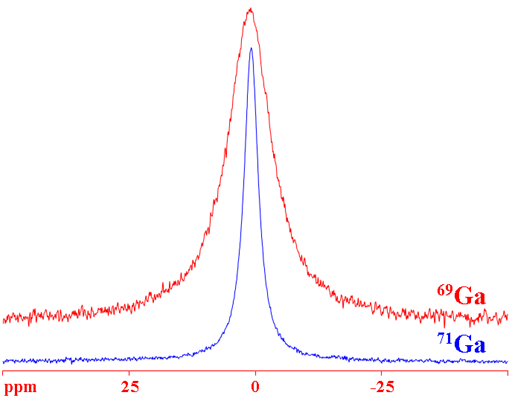
Fig. 2. Chemical shift ranges for gallium NMR
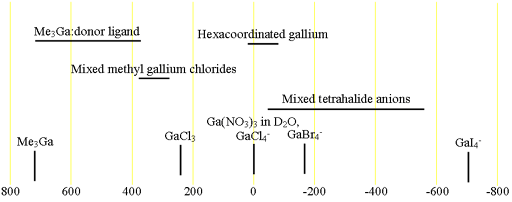
Each type of gallium compound has its characteristic chemical shift range (fig. 2). Note that aqueous solutions of gallium salts are usually hexacoordinated to water.
69Gallium NMR
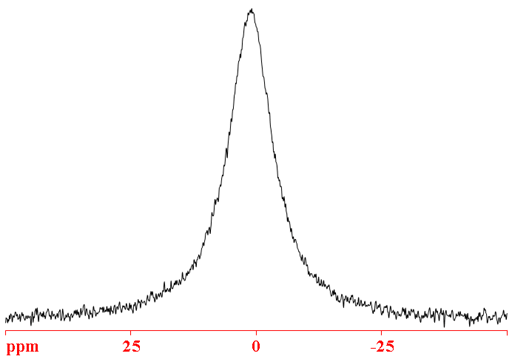
69Ga (fig. 3) is less sensitive and yields broader signals than 71Ga so it is not the nucleus of choice except potentially for certain isotopic enrichment studies.
Fig. 3. 69Ga-NMR spectrum of Ga(NO3)3 (1.1 m) in D2O
Properties of 69Ga
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 60.108% |
| Chemical shift range | 1436 ppm, from -706 to 730 |
| Frequency ratio (Ξ) | 24.001354% |
| Reference compound | Ga(NO3)3 in D2O |
| Linewidth of reference | 1002 Hz |
| T1 of reference | 0.00026 s |
| Receptivity rel. to 1H at natural abundance | 0.0419 |
| Receptivity rel. to 1H when enriched | 0.0697 |
| Receptivity rel. to 13C at natural abundance | 246 |
| Receptivity rel. to 13C when enriched | 409 |
| Linewidth parameter | 390 fm4 |
71Gallium NMR
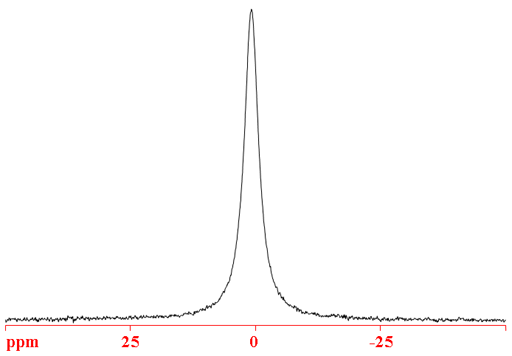
71Ga (fig. 4) is the gallium nucleus of choice as it is more sensitive and yields narrower signals than 69Ga.
Fig. 4. 71Ga-NMR spectrum of Ga(NO3)3 (1.1 m) in D2O
Properties of 71Ga
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 30.83% |
| Chemical shift range | 1436 ppm, from -706 to 730 |
| Frequency ratio (Ξ) | 30.496704% |
| Reference compound | Ga(NO3)3 in D2O |
| Linewidth of reference | 407 Hz |
| T1 of reference | 0.00074 s |
| Receptivity rel. to 1H at natural abundance | 0.0517 |
| Receptivity rel. to 1H when enriched | 0.185 |
| Receptivity rel. to 13C at natural abundance | 335 |
| Receptivity rel. to 13C when enriched | 1087 |
| Linewidth parameter | 150 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, gallium nitrate is an oxidant and irritant and other gallium salts may be corrosive and react violently with water.
References
- B. R. McGarvey, M. J. Taylor and D. G. Tuck, "Gallium-71 NMR studies of anionic gallium halide species in nonaqueous solution" Inorg. Chem., 20, 2010-2013 (1981).
- Z. Cerny, J. Machacek, J. Fusek,O. Kriz, B. Casensky, D. G. Tuck, "71Ga NMR studies of mixtures of gallium trichloride and trimethylgallium" J. Organometal. Chem., 456, 25-30 (1993).