(53Cr) Chromium NMR
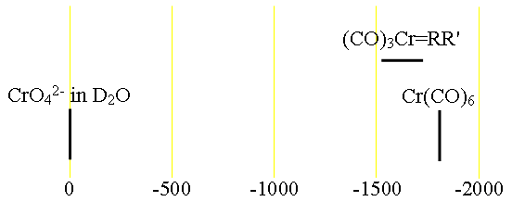
53Chromium (53Cr) is the only NMR active nucleus of chromium. It is a low sensitivity nucleus that yields moderately broad lines in symmetric environments (fig. 1) over a very wide chemical shift range. For larger complexes and molecules, the signals become too broad to observe with a high-resolution NMR spectrometer. Even number oxidation states, such as (0) and (VI) of chromium are diamagnetic while odd number oxidation states are paramagnetic yielding signals much too broad to observe with a high-resolution NMR spectrometer. In most cases, a specific oxidation state of chromium is contaminated with other paramagnetic oxidation states so the NMR of 53Cr is restricted to chromates, carbonyls and carbenes. The Cr(II) oxidation state usually contains too much paramagnetic Cr(III) to yield signals in the high-resolution NMR spectrum. Only in the case of (CO)3Cr carbenes have signals been observed on a high-resolution NMR spectrometer. There is little information available about chemical shifts (fig. 2).
Fig. 1. 53Cr-NMR spectrum of Na2CrO4 (1M) in D2O
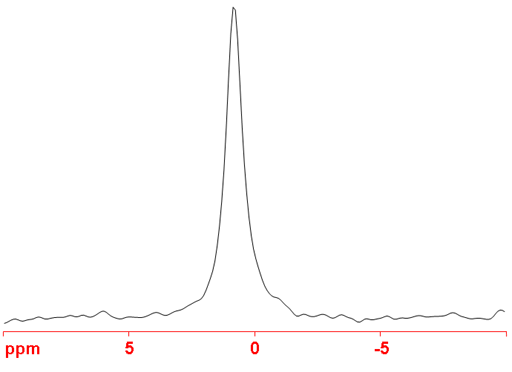
Fig. 2. Chemical shift ranges for chromium NMR
Properties of 53Cr
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 9.501% |
| Chemical shift range | 1795 ppm, from -1795 to 0 |
| Frequency ratio (Ξ) | 5.652496% |
| Reference compound | sat. K2CrO4 in D2O |
| Linewidth of reference | 10 Hz |
| T1 of reference | 0.029 s |
| Receptivity rel. to 1H at natural abundance | 8.63 × 10-5 |
| Receptivity rel. to 1H when enriched | 9.53 × 10-4 |
| Receptivity rel. to 13C at natural abundance | 0.507 |
| Receptivity rel. to 13C when enriched | 5.3 |
| Linewidth parameter | 300 fm4 |
References
- E. Haid, D. Koessler, O. Lutz and W. Schich, J. Magn. Reson., 55, 145 (1983).
- A. Hafnerm, L. S. Hegedus and K. H. Doetz, Adv. Metal Carbene Chem., 247-349 (1989).
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, chromium compounds, including K2CrO4 are toxic carcinogens: wear gloves. Oxidation states VI and VII including K2CrO4 are corrosive.