(25Mg) Magnesium NMR
Use our NMR service that provides 25Mg NMR and many other NMR techniques.
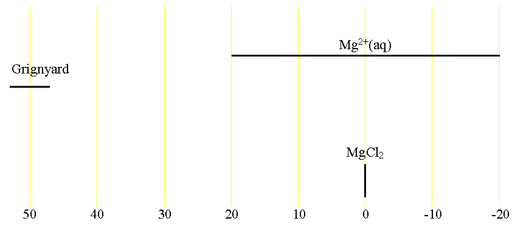
25Magnesium (25Mg) is a low sensitivity nucleus that yields slightly broad lines over a moderate chemical shift range. 25Mg is a spin 5/2 nucleus and is therefore quadrupolar. As a result, the signal width increases with asymmetry of the environment with relatively narrow lines in symmetrical environments (fig. 1) but broad lines in asymmetric ones such as Grignyard reagents. The main use of magnesium NMR is to measure its relaxation rate in order to detect binding to biomolecules. The chemical shift of inorganic magnesium is quite solvent dependent but there is little information available about magnesium chemical shifts (fig. 2).
Fig. 1. 25Mg-NMR spectrum of MgCl2 in D2O
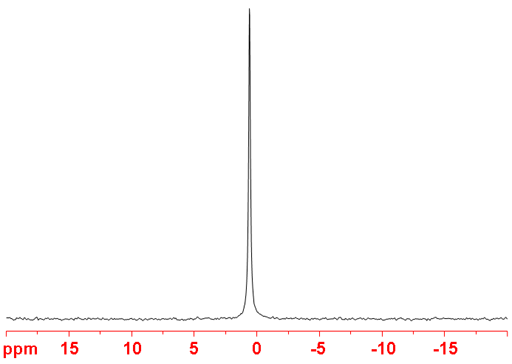
Fig. 2. Chemical shift ranges for 25Mg NMR
Properties of 25Mg
| Property | Value |
|---|---|
| Spin | 5/2 |
| Natural abundance | 10.00% |
| Chemical shift range | 70 ppm, from -20 to 50 |
| Frequency ratio (Ξ) | 6.121635% |
| Reference compound | 11 M MgCl2 in D2O |
| Linewidth of reference | 2.3 Hz |
| T1 of reference | 0.13 s |
| Receptivity rel. to 1H at natural abundance | 2.68 × 10-4 |
| Receptivity rel. to 1H when enriched | 2.68 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 1.58 |
| Receptivity rel. to 13C when enriched | 15.8 |
| Linewidth parameter | 130 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, magnesium salts can be toxic in large doses.