(9Be) Beryllium NMR
9Beryllium NMR is mostly used to study coordination complexes of beryllium. Each type of signal has a characteristic chemical shift range (fig. 1). The chemical shift ranges for beryllium are generally indicative of the coordination order and type and can, in principle, be used to determine the coordination type. The reference compound, BeSO4 is actually a 4-coordinated complex [Be(D2O)4]SO4 and resonates at 0 ppm, within the 4-coordinated range.
Fig. 1. Chemical shift ranges for beryllium NMR
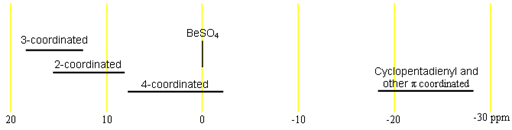
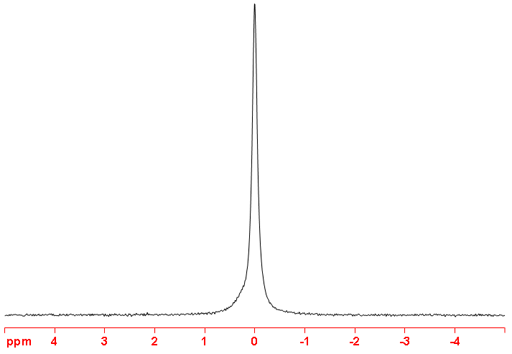
9Be is a quadrupolar nucleus so yields broad lines, up to several hundred Hertz, for large complexes with reduced symmetry. However, it can yield lines several Hertz wide for small symmetric complexes such as [Be(H2O)4]2+ (fig. 2).
Fig. 2. 9Be-NMR spectrum of Be(SO4) (0.43 m) in D2O
Properties of 9Be
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 100% |
| Chemical shift range | 46 ppm, from -18 to 28 |
| Frequency ratio (Ξ) | 14.051813% |
| Reference compound | 0.43 m BeSO4 in D2O |
| Linewidth of reference | 7 Hz |
| T1 of reference | 1.3 s |
| Receptivity rel. to 1H at natural abundance | 0.0139 |
| Receptivity rel. to 1H when enriched | 0.0139 |
| Receptivity rel. to 13C at natural abundance | 81.5 |
| Receptivity rel. to 13C when enriched | 81.5 |
| Linewidth parameter | 37 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Beryllium compounds are very toxic. Be especially careful not to inhale or ingest powders and to avoid eye contact.