(29Si) Silicon NMR
Use our NMR service that provides 29Si NMR and many other NMR techniques.
29Silicon NMR is a low sensitivity nucleus which has spin ½ and yields sharp lines (fig. 1). Where there is heteronuclear coupling to proton, the spectrum is usually decoupled.
Fig. 1. 29Si NMR spectrum of TMS
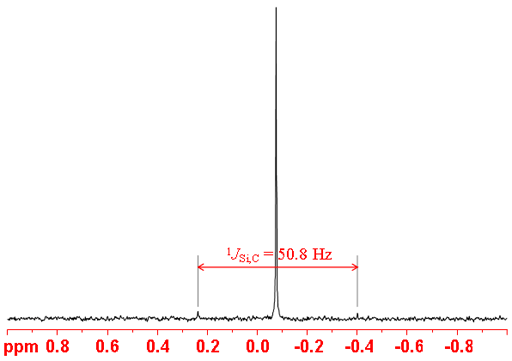
Silicon has a wide chemical shift range (fig. 2) so it is good for determining the chemical environment in silicon compounds.
Fig. 2. Chemical shift ranges for 29Si NMR
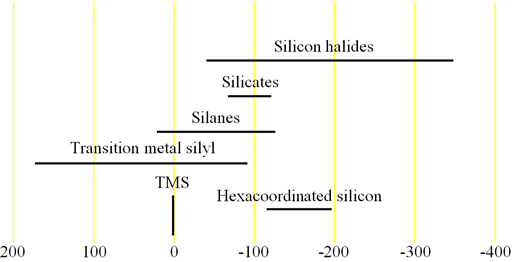
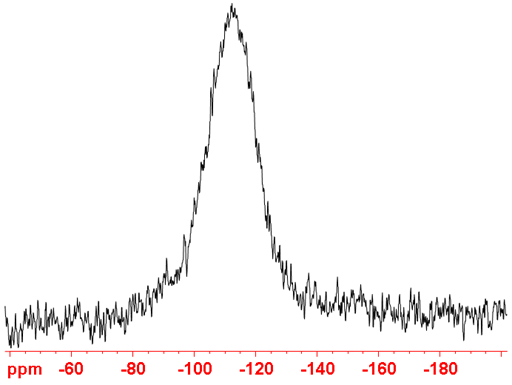
The spectrum always contains a broad background signal from the glass and quartz in the tube and probe at -110 ppm (fig. 3). This can be suppressed by running a blank spectrum and subtracting that from the regular spectrum. The disadvantage of this is that it is time-consuming. If the sample contains broad signals in the same region such as a dilute solution of silicate gels then this method will not suffice. An alternative relatively inexpensive solution (you may damage the probe so try it at your own risk) is to remove the quartz tubing from the probe and use a Teflon NMR tube. The best solution is to replace the quartz in the probe with sapphire and use sapphire NMR tubes however, this is very expensive and such a probe cannot be used for 27Al-NMR. Another solution is to use a zircon HR-MAS rotor in a CP-MAS probe although this reduces sensitivity and resolution it is suitable for small quantities of concentrated solutions that yield broad signals at similar chemical shifts to glass.
Fig. 3. Background 29Si signal arising from glass and quartz
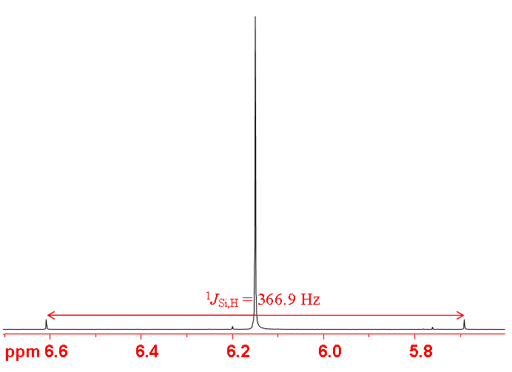
Silicon couples with protons with a one bond-heteronculear coupling constant of hundreds of Hertz (figs. 4, 5). However, silicon-proton bonds are usually highly unstable to air and moisture so are not observed in most applications.
Fig. 4. 29Si-NMR spectrum of SiHCl3 showing one-bond coupling to proton
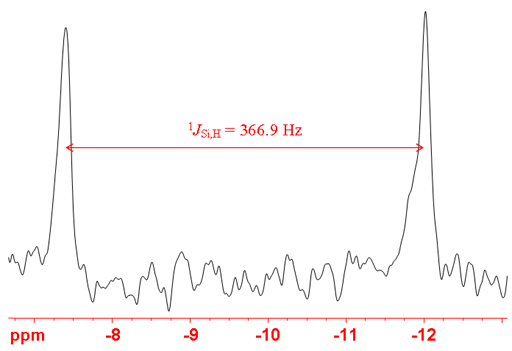
Fig. 5. 1H-NMR spectrum of SiHCl3 showing one-bond coupling to the 29Si satellites
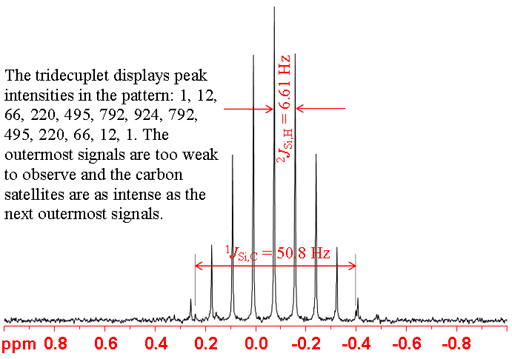
More commonly, 2-bond couplings, typically of 6.6 Hz are observed in alkyl silanes. An extreme example of this is TMS where the silicon is coupled to 12 protons yielding a tridecuplet (13 peaks) (fig. 6). Advantage can be taken of these two bond-couplings to improve sensitivity by using the projection of a heteronuclear correlation.
Fig. 6. Fully coupled 29Si-NMR spectrum of TMS in CDCl3
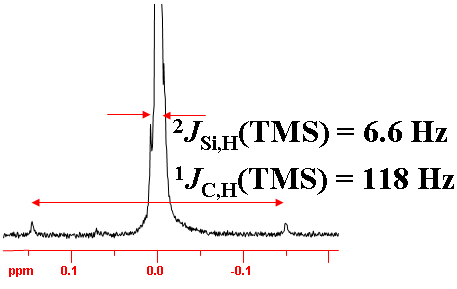
Likewise two-bond silicon coupling can be observed in the 1H spectrum (fig. 7).
Fig. 7. 1H signal of TMS showing 13C and 29Si couplings as satellite signals
Properties of 29Si
| Property | Value |
|---|---|
| Spin | ½ |
| Natural abundance | 4.6832% |
| Chemical shift range | 519 ppm, from -346 to 173 |
| Frequency ratio (Ξ) | 19.867187% |
| Reference compound | Dilute TMS in CDCl3 |
| Linewidth of reference | 0.15 Hz |
| T1 of reference | 5 s |
| Receptivity rel. to 1H at natural abundance | 3.68 × 10-4 |
| Receptivity rel. to 1H when enriched | 7.86 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 2.16 |
| Receptivity rel. to 13C when enriched | 46.1 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, silica hydrides are very reactive and many organosilicon compounds are toxic and flammable.