(Te) Tellurium NMR
Use our NMR service that provides Te NMR and many other NMR techniques.
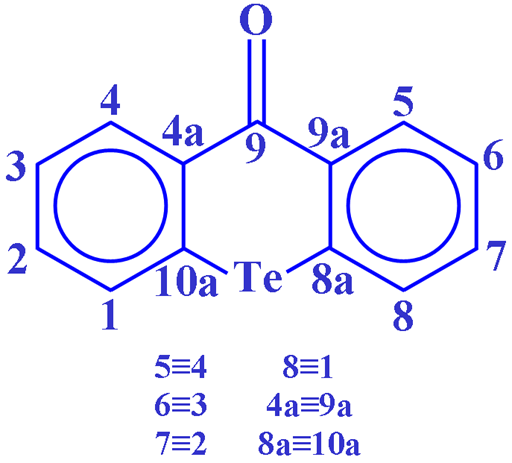
Tellurium has two medium to low sensitivity spin ½ nuclei that yield narrow lines over a very wide chemical shift range. 125Te has a higher sensitivity and may yield sharper signals than 123Te (fig. 1) so 125Te is the tellurium nucleus of choice. Tellurium NMR is used for the study of organotellurium compounds, such as telluroxanthenone (fig. 2), their structure, symmetry and dynamics as well as inorganic tellurium compounds.
Fig. 1. Comparison of 123Te and 125Te NMR of telluroxanthenone
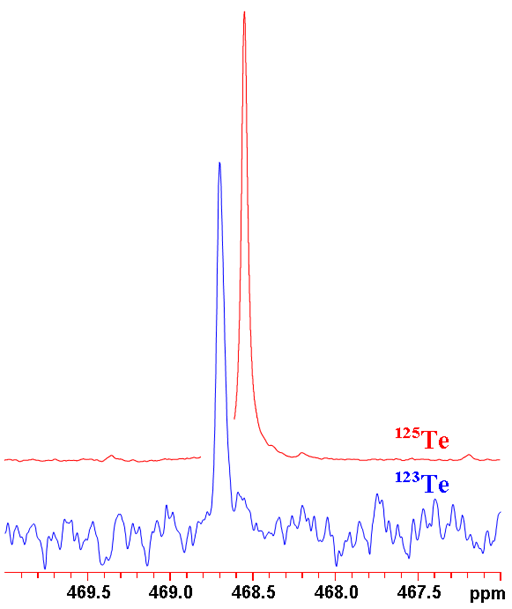
Fig. 2. Molecular sructure of telluroxanthenone
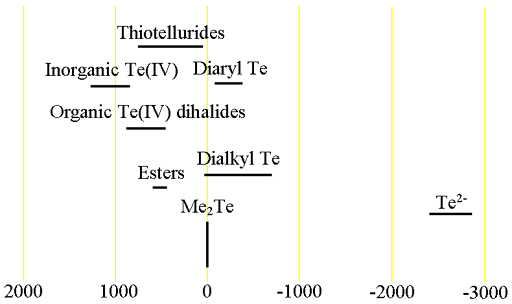
Each type of tellurium compound has its characteristic chemical shift range (fig. 2).
Fig. 3. Chemical shift ranges for tellurium NMR
123Tellurium NMR
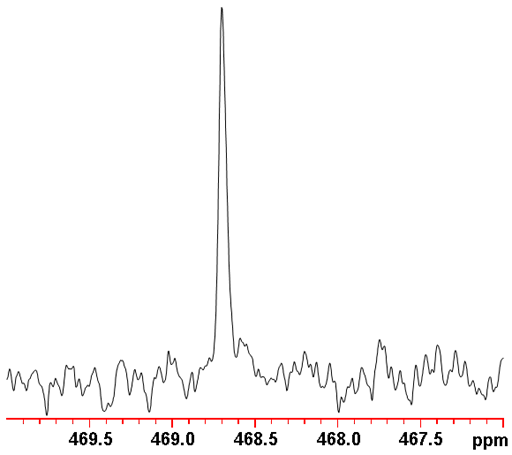
123Te NMR is less sensitive and yields slightly broader signals (fig. 4) than 125Te NMR. Therefore, 123Te NMR is not the tellurium nucleus of choice.
Fig. 4. Proton decoupled 123Te spectrum of telluroxanthenone (0.1 M) in CDCl3
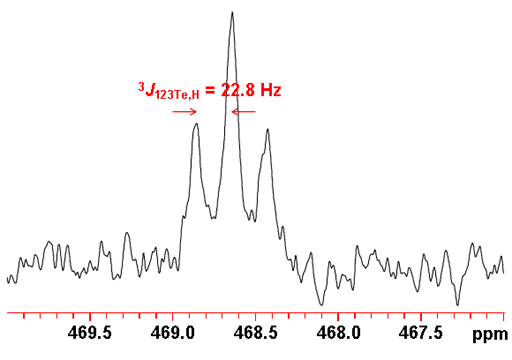
Tellurium often shows couplings to other nuclei, 1H, 13C, 31P, etc. Tellurium couplings to protons can be removed by decoupling as in the spectrum above. The coupled spectrum of telluroxanthenone (fig. 5) shows a three bond coupling to H1 (2J123Te,H) of 22.8 Hz. Longer range couplings are not resolved.
Fig. 5. 123Te spectrum of telluroxanthenone (0.1 M) in CDCl3 showing proton coupling
Properties of 123Te
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 0.89% |
| Chemical shift range | 5800 ppm, from -1400 to 3400 |
| Frequency ratio (Ξ) | 26.169742% |
| Reference compound | Me2Te (90%) in C6D6 |
| Linewidth of reference | ~3 Hz |
| T1 of reference | ~2 s |
| Receptivity rel. to 1H at natural abundance | 1.64 × 10-4 |
| Receptivity rel. to 1H when enriched | 0.018 |
| Receptivity rel. to 13C at natural abundance | 0.961 |
| Receptivity rel. to 13C when enriched | 108 |
125Tellurium NMR
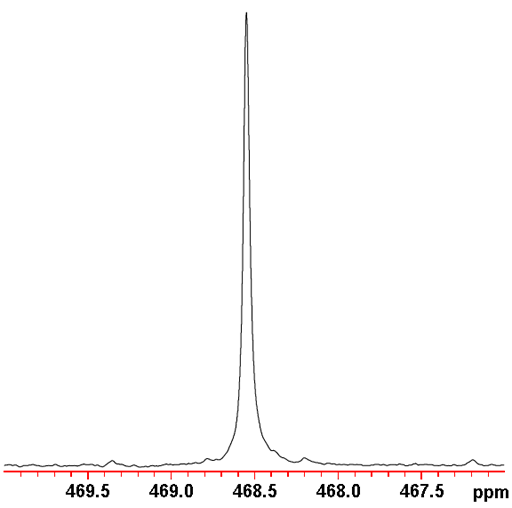
125Te NMR is more sensitive and yields slightly sharper signals (fig. 6) that 123Te NMR. Therefore, 125Te NMR is the tellurium nucleus of choice.
Fig. 6. Proton decoupled 125Te spectrum of telluroxanthenone (0.1 M) in CDCl3
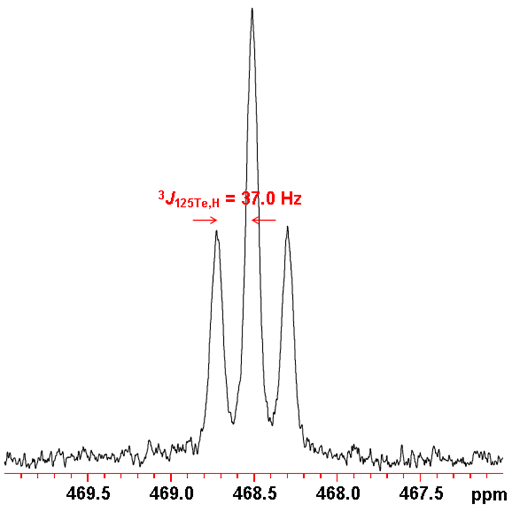
Tellurium often shows couplings to other nuclei, 1H, 13C, 31P, etc. Tellurium couplings to protons can be removed by decoupling as in the spectrum above. The coupled spectrum of telluroxanthenone (fig. 7) shows a three bond coupling to H1 (2J125Te,H) of 37.0 Hz. Longer range couplings are not resolved.
Fig. 7. 125Te spectrum of telluroxanthenone (0.1 M) in CDCl3 showing proton coupling
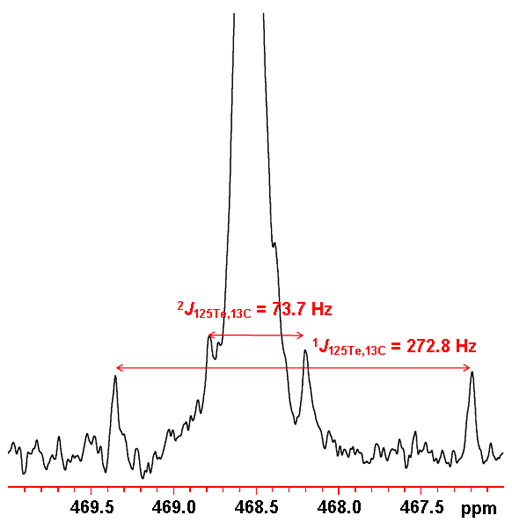
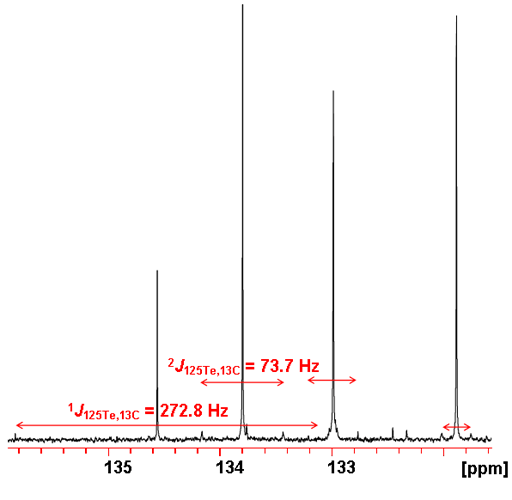
The tellurium couplings are also observed as slightly broad satellites in the proton spectrum and are easily confused with impurity signals. They only appear as satellites because the abundance of 125Te is only 7.07%. The abundance of 123Te is so low that its coupling is not noticeable in the 1H spectrum. 13C coupling is manifested as small satellite signals in the 125Te spectrum (fig. 8). Here couplings up to three bonds are observed. One bond coupling constants are in the region of 270 Hz, 2 and 3 bond couplings range from 10 to 75 Hz and longer range couplings are less than 10 Hz.
Fig. 8. 125Te spectrum of telluroxanthenone (0.1 M) in CDCl3 showing satellites arising from 13C coupling
Tellurium carbon coupling is also seen as satellite signals in the 13C NMR spectrum (fig. 9).
Fig. 9. Proton decoupled 13C spectrum of telluroxanthenone (0.1 M) in CDCl3 showing satellites arising from 125Te coupling
Properties of 125Te
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 7.07% |
| Chemical shift range | 5800 ppm, from -1400 to 3400 |
| Frequency ratio (Ξ) | 31.549769% |
| Reference compound | Me2Te (90%) in C6D6 |
| Linewidth of reference | 1.5 Hz |
| T1 of reference | ~2 s |
| Receptivity rel. to 1H at natural abundance | 2.28 × 10-3 |
| Receptivity rel. to 1H when enriched | 0.0322 |
| Receptivity rel. to 13C at natural abundance | 13.4 |
| Receptivity rel. to 13C when enriched | 190 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, tellurium compounds are toxic (LD50 2.5 mg): wear protective gloves. Dimethyl tellurium is volatile and stinks: wear protective clothing and work in a hood. Latex gloves do not provide protection
References
- B. Kohne, W. Lohner, K. Praefcke, H. J. Jakobsen and B. Villadsen, "Spectroscopic investigations XVII. 77Se and 125Te NMR resonances of some selenol and tellurol esters", J. Organometal. Chem., 166, 373-377 (1979).
- W. W. Du Mont and H. J. Kroth, "Tellurium-125 NMR shifts and Te-P coupling constants of phosphine tellurides, tellurophosphines, and tellurophosphine complexes" Z. Natur. B, 36B, 332-334 (1981).
- D. H. OwBrien, N. Dereu, R. A. Grigsby, K. J. Irgolic and F. F. Knapp Jr., "Tellurium-125 chemical shifts of symmetric and unsymmetric dialkyl ditellurides", Organometal., 1, 513-517 (1982).
- W. Mazurek, A. G. Moritz and M. J. O'Connor, "Tellurium-125 NMR and mass spectra of dithiotellurides", Inorg. Chim. Acta, 113, 143-146 (1986).
- M. R. Detty, W. C. Lenhart, P. G. Gassman and M. R. Callstrom, "X-ray photoelectron spectroscopy and tellurium-125 NMR studies of organotellurium compounds. II. Oxatellurolylium halides and dioxatellurapentalenes and theis products of oxidative halogen addition", Organometal., 8, 866-870 (1989).
- B. Bildstein, K. J. Irgolic and D. H. Orien, "A tellurium-125 study of lithium alkane- and arentellurolates", Phosphorus and Sulfur and the Related Elements, 38, 245-256 (1987).
- W. A. Herrmann and H. J. Kneuper, "Multiple bonds between main group elements and transition metals. LIII. Tellurium-125 and selenium-77 NMR of transition metal complexes with naked tellurium or selenium bridging ligands", J. Organometal. Chem., 348, 193-197 (1988).
- W. Nakanishi, S. Hayashi, H. Tukada and H. Iwamura, "Structural studies of halogen adducts of diorganyl chalcogenides in solutions by proton, carbon-13, selenium-77 and tellurium-125 NMR", J. Phys. Org. Chem., 3, 358-368 (1990).
- L. A. Silks III, J. D. Odom and R. B. Dunlap, "Synthesis and 125-tellurium NMR spectroscopy of α-tellurocarbonyl compounds and derivatives", Synth. Comm., 21, 1105-1119 (1991).
- R. U. Kirss and D. W. Brown, "Ligand-exchange reactions on organotellurides by 125Te NMR spectroscopy", Organometal., 10, 3597-3599 (1991).
- H. Duddeck and A, Bialiass, "Substituent effects and stereochemistry in 125Te NMR spectroscopy. Diorganyltellurium dihalides and some tellurides and ditellurides", Magn. Reson. Chem., 32, 303-311 (1994).
- I. P. dA. Campos, H. A. Stefani, L. C. Roque, M. A. Montoro and A. L. Braga, "The γ-cis effect in the tellurium-125 nuclear magnetic resonance spectroscopy of susbstituted vinylic tellurides", J. Chem. Res., Syn., 112-125 (1995).
- A. Levy, U. P. Biedermann, S. Cohen and I. Agranat, "Selenium and tellurium tricycles. Conformational effects on 77Se and 125Te NMR spectra", Phosphorus Sulfur Silicon Rel. Elem., 136-138, 139-142 (1998).
- S. Saito, J. Zhang, K. Tanida, S. Takahashi and T. Koizumi, "A systematic 125Te NMR study of organotellurium compounds: the effect of oxidation states and substituents", Tetrahed., 55, 2545-2552 (1999).