31Phosphorus NMR
Use our NMR service that provides 31P NMR and many other NMR techniques.
The 1D 31Phosphorus NMR experiment is much less sensitive than Proton (1H) but more sensitive than 13Carbon. 31Phosphorus is a medium sensitivity nucleus that yields sharp lines (fig. 1) and has a wide chemical shift range. It is usually acquired with 1H decoupling (fig. 2) means that spin-spin couplings are seldom observed. This greatly simplifies the spectrum and makes it less crowded. Where there are one-bond 31P-1H couplings present then the decoupling power needs to be at least twice that needed for 13C because of the large coupling constant.
Fig. 1. Typical 31P-NMR spectrum of a mixture of organic phosphates
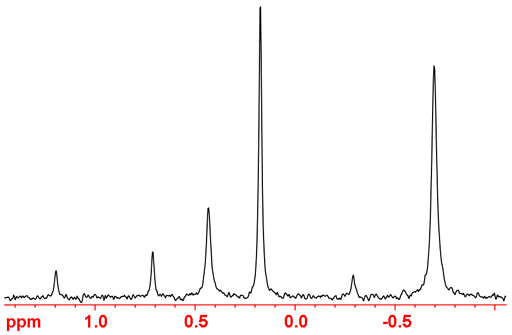
Integration is inaccurate (almost useless when there is one-bond coupling to 1H) in a regular decoupled 31P NMR spectrum because of uneven NOE enhancement of the signals by decoupling and long longitudinal relaxation times (T1's). Quantitative spectra may be obtained by inverse gated decoupling.
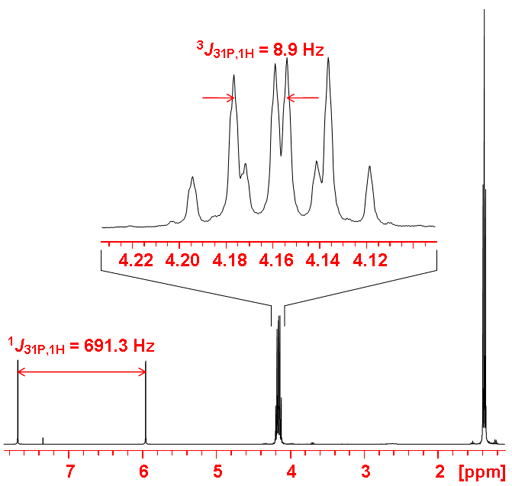
Gated decoupling may be used in order to observe proton couplings (fig. 4). If coupling constants are required then a phosphorus coupled proton spectrum (fig. 5) is much more sensitive than the phosphorus spectrum although the reduced chemical shift range may cause overlapping that make analysis more difficult. One-bond couplings are typically 600 to 700 Hz, two-bond 20 to 30 Hz, three-bond 5 to 10 Hz and four-bond < 1 Hz. Couplings may be observed with other nuclei such as 19Fluorine.
Fig. 2. 31P-NMR spectrum of diethyl phosphonate with 1H decoupling
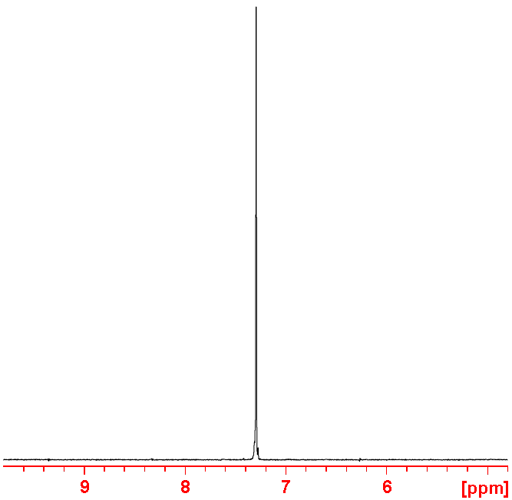
Fig. 3. Molecular structure of diethyl phosphonate
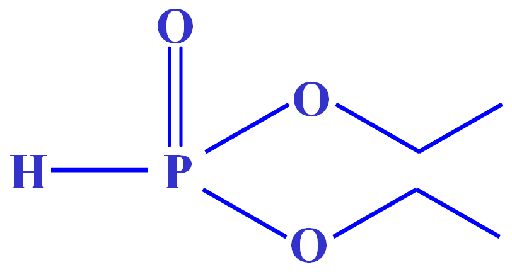
Fig. 4. Spectrum of diethyl phosphonate showing one-bond and three-bond coupling to 1H
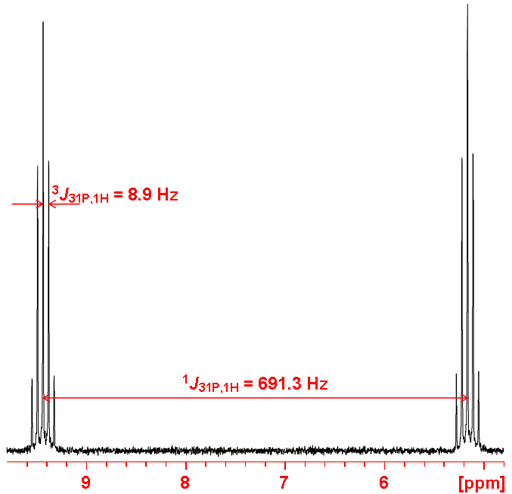
Fig. 5. 1H-NMR spectrum of diethyl phosphonate showing coupling to 31P
A typical analysis of a 31P NMR spectrum consists of matching expected chemical shifts to the expected moieties. Each type of signal has a characteristic chemical shift range (fig. 6).
Fig. 6. Chemical shift ranges of phosphorus according to their chemical environment
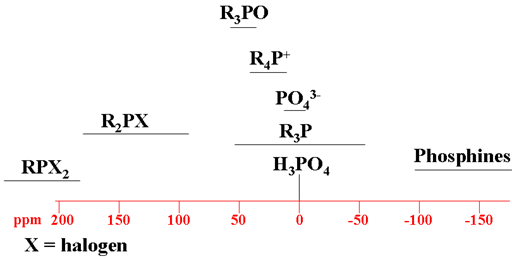
Choose the structure that most closely represents the phosphorus in question. R = alkyl or H.
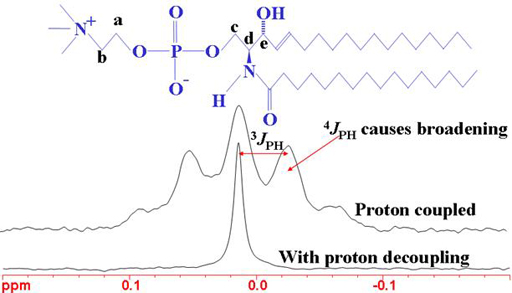
The heteronuclear coupling patterns between phosphorus and proton can be used to assign the proton spectrum (figs. 7, 8).
Fig. 7. 1H-NMR spectrum of sphingomyelin showing the effects of 31P coupling
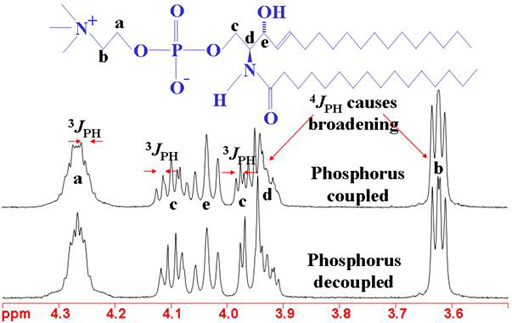
Fig. 8. 31P-NMR spectrum of sphingomyelin showing the effects of 1H coupling
Properties of 31P
| Property | Value |
|---|---|
| Spin | ½ |
| Natural abundance | 100% |
| Chemical shift range | 430 ppm, from -180 to 250 |
| Frequency ratio (Ξ) | 40.480742% |
| Reference compound | 85% H3PO4 in H2O = 0 ppm |
| Linewidth of reference | 1 Hz |
| T1 of reference | 0.5 s |
| Receptivity rel. to 1H at natural abundance | 6.63 × 10-3 |
| Receptivity rel. to 1H when enriched | 6.63 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 377 |
| Receptivity rel. to 13C when enriched | 377 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Phosphoric acid, phosphines and phosphorus halides are toxic and irritant: wear protective gloves.