(La) Lanthanum NMR
Use our NMR service that provides La NMR and many other NMR techniques.
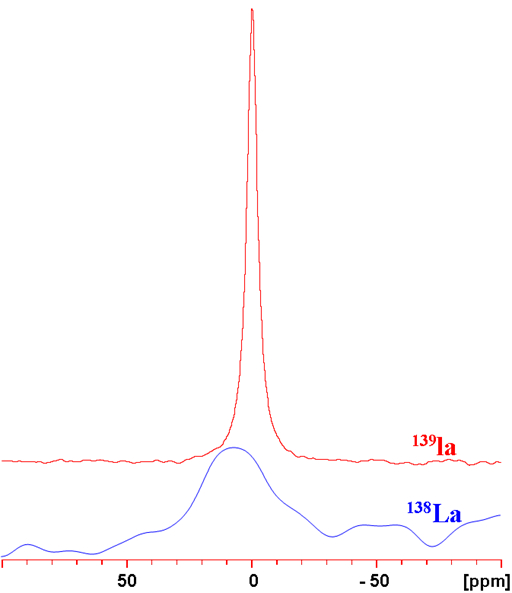
Lanthanum (La) has two quadrupolar NMR active nuclei 138La and 139La (fig. 1). They yield broad signals in symmetric environments and very broad signals in small complexes. The signals from larger complexes are too broad to be observed with a high-resolution NMR spectrometer. 139La is medium sensitivity nucleus and much more sensitive than the very low sensitivity 138La. 138La also yields broader signals. Therefore 139La is the lanthanum nucleus of choice. Lanthanum has a very wide chemical shift range. Lanthanum NMR is used for studying small lanthanum complexes and its relaxation rate is used in studies of binding.
Fig. 1. Comparison of 138La and 139La NMR for LaCl3 (0.01 M) in D2O. The experimental conditions were different for each nucleus so the 139La signal should be about 1000 times more sensitive than it appears as compared with the 138La signal.
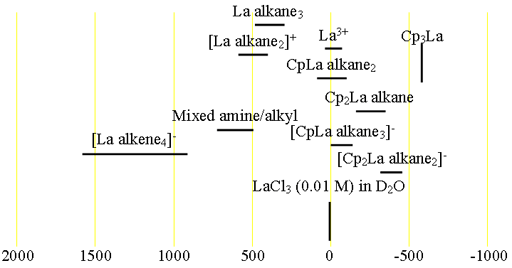
Each type of lanthanum has its characteristic chemical shift range (fig. 2).
Fig. 2. Chemical shift ranges for lanthanum NMR
138Lanthanum NMR
138La (fig. 3) yields signals that are broader and much less sensitive and than 139La. Therefore 138La is not the lanthanum nucleus of choice for NMR.
Fig. 3. 138La NMR spectrum of LaCl3 (0.01 M) in D2O

Properties of 138La
| Property | Value |
|---|---|
| Spin | 5 |
| Natural abundance | 0.090% |
| Chemical shift range | 2200 ppm, from -600 to 1600 |
| Frequency ratio (Ξ) | 13.194300% |
| Reference compound | 0.01 M LaCl3 in D2O |
| Linewidth of reference | 700 Hz |
| T1 of reference | 0.001 s |
| Receptivity rel. to 1H at natural abundance | 8.46 × 10-5 |
| Receptivity rel. to 1H when enriched | 0.0940 |
| Receptivity rel. to 13C at natural abundance | 0.497 |
| Receptivity rel. to 13C when enriched | 552 |
| Linewidth parameter | 120 fm4 |
139Lanthanum NMR
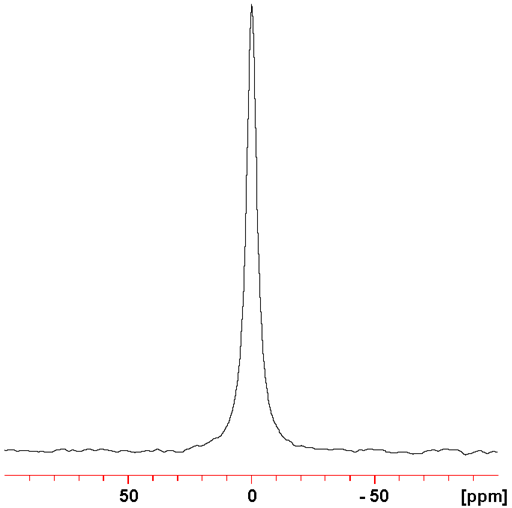
139La (fig. 4) yields signals that are less broad and much more sensitive and than 138La. Therefore 139La is the lanthanum nucleus of choice for NMR.
Fig. 4. 139La NMR spectrum of LaCl3 (0.01 M) in D2O
Properties of 139La
| Property | Value |
|---|---|
| Spin | 7/2 |
| Natural abundance | 99.91% |
| Chemical shift range | 2200 ppm, from -600 to 1600 |
| Frequency ratio (Ξ) | 14.125641% |
| Reference compound | 0.01 M LaCl3 in D2O |
| Linewidth of reference | 142 Hz |
| T1 of reference | 0.0023 s |
| Receptivity rel. to 1H at natural abundance | 0.0605 |
| Receptivity rel. to 1H when enriched | 0.0606 |
| Receptivity rel. to 13C at natural abundance | 356 |
| Receptivity rel. to 13C when enriched | 356 |
| Linewidth parameter | 54 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, lanthanum salts may be toxic besides toxicity arising from the anion: wear protective gloves.
References
- O. Lutz, and H. Oehler, "Lanthanum-138 and lanthanum-139 nuclear magnetic resonance studies", J. Magn. Reson., 37, 261-267 (1980).
- V. P. Tarasov, G. A. Kirakosyan, Y. A. Buslaev, S. V. Trots, and V. T. Panyushkin, "Lanthanum-139 NMR study of aqueous dimethylformamide solutions", Koordinat. Khim., 11, 913-917 (1985).
- S. H. Eggers, and R. D. Fischer, "Preparation and lanthanum-139 NMR characterization of tris(cyclopentadienyl)bis(cyclohexyl isonitrile) lanthanum (III)", J. Organometal. Chem., 315, C61-C63 (1986).
- M. Adam, E. T. K. Haupt, and R. D. Fischer, "Lanthanum-139 NMR spectroscopy of organolanthanum(III) complexes. Part IV. Lanthanum-139 NMR : investigations on organolanthanum compounds", Bull. Magn. Reson., 12, 101-103 (1990).
- A. F. Savost'yanova, V. V. Trachevskii, and V. S. Kuts, "Magnetic resonance characteristics of lanthanum dithiocarbamates" Koordinat. Khim., 17, 417-421 (1991).
- H. Windisch, J. Scholz, R. Taube and B. Wrackmeyer, "139La-NMR-spectroscopy of allyl lanthanum (III) complexes", J. Organometal. Chem., 520, 23-30 (1996).
- Y. Israeli, and C. Detellier, "Multinuclear magnetic resonance study of the complexation of lanthanum (III) by D-glucitol and ribitol in aqueous solution", Carbohyd. Res., 297, 201-207 (1997).
- T. Yaita, D. Ito and S. Tachimori, "139La NMR relaxation and chemical shift studies in aqueous nitrate and chloride solutions", J. Phys. Chem. B, 102, 3886-3891 (1998).