(K) Potassium NMR
Use our NMR service that provides K NMR and many other NMR techniques.
Potassium (K) has three NMR active nuclei, 39K, 40K and 41K (fig. 1), that are all quadrupolar and yields slightly to very broad lines over a small chemical shift range. Usually 39K is preferred as it has the highest sensitivity and yields sharper signals than 41K. Although 40K yields sharper signals than 39K, it has such a low sensitivity that its use is severely restricted. Potassium NMR is mostly used for detecting binding in aqueous solutions by measureing its relaxation rate. However, some chemical shift information of organic potassium compounds is obtainable.
Fig. 1. Comparison of the potassium isotopes under comparable conditions for KNO2 (~15 M) in D2O
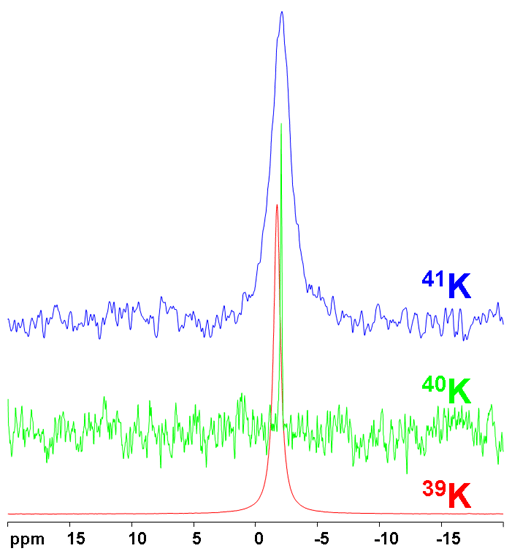
All the isotopes have the same chemical shifts (fig. 2) although there is little information available about these shifts.
Fig. 2. Chemical shift ranges for potassium NMR

39Potassium NMR
(39K) 39Potassium is a spin 3/2 nucleus and is therefore quadrupolar. As a result, the signal width increases with asymmetry of the environment. Hence, in the fig. 3, KCl is narrower than KOMe. 39K NMR is mostly used for detecting binding in aqueous solutions by measureing its relaxation rate. However, some chemical shift information of organic potassium compounds is obtainable.
Fig. 3. 39K-NMR spectra showing increasing linewidth with increasing environmental asymmetry

29K has a low intrinsic sensitivity although it has a high natural abundance. Although it yields broader signals than 40K, it is the nucleus of choice because it is the much more sensitive and for most applications, resolution is not the overriding issue.
Properties of 39K
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 95.2381% |
| Chemical shift range | 65 ppm, from -30 to 35 |
| Frequency ratio (Ξ) | 4.666373% |
| Reference compound | 0.1 M KCl in D2O |
| Linewidth of reference | 5 Hz |
| T1 of reference | 0.049 s |
| Receptivity rel. to 1H at natural abundance | 4.76 × 10-4 |
| Receptivity rel. to 1H when enriched | 5.00 × 10-4 |
| Receptivity rel. to 13C at natural abundance | 2.79 |
| Receptivity rel. to 13C when enriched | 2.93 |
| Linewidth parameter | 46 fm4 |
40Potassium NMR
(40K) 40Potassium is a spin 4 nucleus and is therefore quadrupolar. As a result, the signal width increases with asymmetry of the environment. 40K has a medium intrinsic sensitivity but has a very low natural abundance. It is also slightly radiocative so, although this is not a safety issue at natural abundance, enrichment could lead to safety issues in addition to the high expense of enrichment. Although 40K yields sharper signals (fig. 4) than 39K, it has never been reported as the nucleus of choice because it is the much less sensitive and for most applications, resolution is not the overriding issue.
Fig. 4. 40K-NMR spectrum of KNO2 (~15 M) in D2O
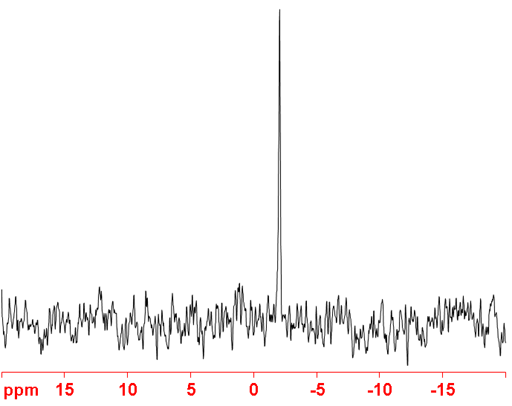
Properties of 40K
| Property | Value |
|---|---|
| Spin | 4 |
| Natural abundance | 0.0117% |
| Chemical shift range | 65 ppm, from -30 to 35 |
| Frequency ratio (Ξ) | 5.802018% |
| Reference compound | 0.1 M KCl in D2O |
| Linewidth of reference | 2 Hz |
| T1 of reference | 0.3 s |
| Receptivity rel. to 1H at natural abundance | 6.12 × 10-7 |
| Receptivity rel. to 1H when enriched | 5.23 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 3.59 × 10-3 |
| Receptivity rel. to 13C when enriched | 30.7 |
| Linewidth parameter | 5.2 fm4 |
41Potassium NMR
(41K) 41Potassium is a spin 3/2 nucleus and is therefore quadrupolar. As a result, the signal width increases with asymmetry of the environment. 41K NMR is not the preferred potassium nucleus as it yields broader signals (fig. 5) than both 39K and 40K and is less sensitive than 39K. The most likely application for this nucleus is for measuring isotopic enrichment of potassium.
Fig. 5. 41K-NMR spectrum of KNO2 (~15 M) in D2O

Properties of 41K
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 6.7302% |
| Chemical shift range | 65 ppm, from -30 to 35 |
| Frequency ratio (Ξ) | 2.561305% |
| Reference compound | 0.1 M KCl in D2O |
| Linewidth of reference | 12 Hz |
| T1 of reference | 0.01 s |
| Receptivity rel. to 1H at natural abundance | 5.68 × 10-6 |
| Receptivity rel. to 1H when enriched | 8.43 × 10-5 |
| Receptivity rel. to 13C at natural abundance | 0.0333 |
| Receptivity rel. to 13C when enriched | 0.495 |
| Linewidth parameter | 67 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, potassium salts are non-toxic on account of the potassium under unless ingested or injected in decagram quantities, however, they may be toxic on account of their anion as is the case for KOCD3 and to a lesser extent KNO2 mentioned below. 40K is slightly radioactive. If isotopically enriched this may pose a radiation hazard.