1D Incredible natural abundance double quantum transfer experiment (INADEQUATE)
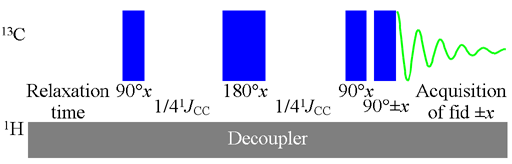
Couplings to other carbons yield very weak signals at natural abundance and appear as small satellites in very concentrated samples or larger satellites in 13C enriched samples. The one-bond coupling constants can be used to estimate carbon-carbon bond order. The constants are usually 35 to 45 for a single bond and about 65 Hz for a double bond and are increased by electronegative substituents. Often the peak overlap obscures the 13C satellites (as in the right-hand peaks in the figure below) in which case the coupling constants can be measured using a 1D-INADEQUATE (incredible natural abundance double quantum transfer experiment). INADEQUATE suppresses the main uncoupled signal and yields only the satellites as antiphase (one up and one down) multiplets (fig. 1). However, INADEQUATE requires enriched or very concentrated samples and careful pulse calibration. In principle, the INADEQUATE experiment could be used for assignment by comparing coupling constants and looking for AB type roofing effects, but unless there are no protons near the carbons, there are much more sensitive assignment techniques available. Fig. 1 shows the 1D INADEQUATE spectrum of cholesteryl acetate.
Fig. 1. 1D INADEQUATE spectrum of 90% cholesteryl acetate in CDCl3. The upper regular 13C spectrum shows the 13C satellites. The lower spectrum is a 1D INADEQUATE showing antiphase doublets
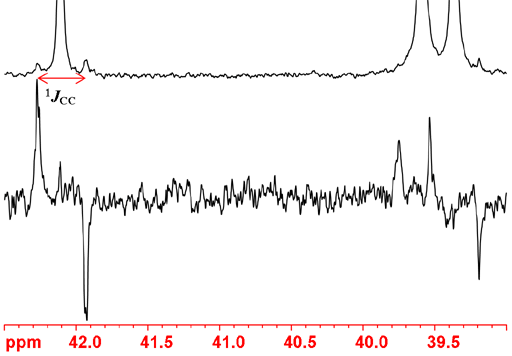
The 1D INADEQUATE pulse sequence is shown in fig. 2. The delay between the pulses is set to one over four times the carbon carbon coupling constant. This is usually about 40 Hz so the delay is 6.25 ms. Between the last two pulses and between the last pulse and the acquisition 3μs is usually sufficient.
Fig. 2. Pulse sequence for 1D INADEQUATE