Boron NMR
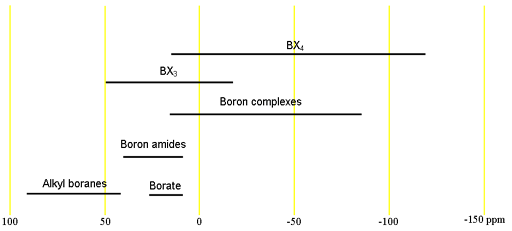
Boron has two naturally occurring NMR active nuclei. Both nuclei have spins of greater than ½ and are quadrupolar. 11B has a spin of 3/2 and 10B is spin 3. 11B is the better nucleus in all respects, having the lower quadrupole moment and being more sensitive. Regular NMR tubes are made of borosilicate glass and therefore contain boron. As a result there is a broad signal in the spectrum arising from the tube. It is therefore preferable to use quartz tubes that do not contain boron although these are much more expensive and fragile than regular tubes. Both nuclei have the same wide chemical shift range. Each type of signal has a characteristic chemical shift range (fig. 1) which is the same for both nuclei.
Fig. 1. Chemical shift ranges for boron NMR
10Boron
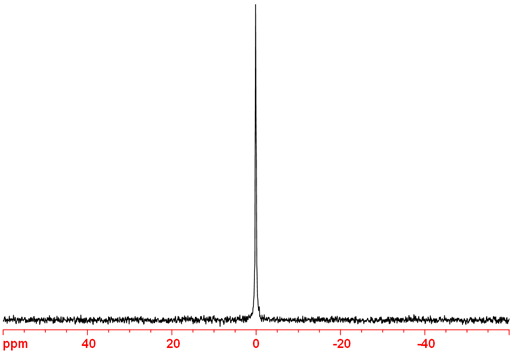
10B-NMR (fig. 2) has a lower sensitivity and yields broader signals than 11B. It is therefore preferable to use 11B unless the sample is enriched in 10B as may be the case for samples intended for neutron capture applications.
Fig. 2. 10B-NMR spectrum of BF3.OEt2 in CDCl3
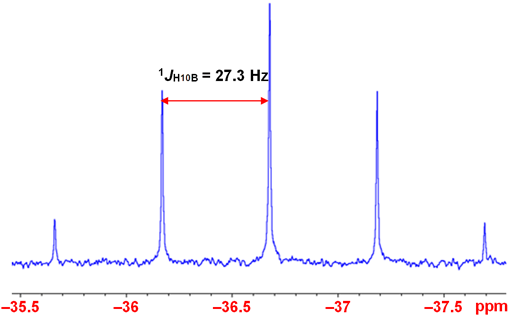
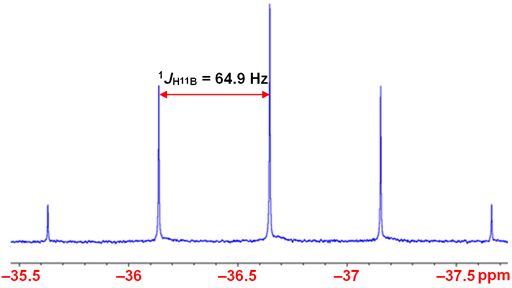
Coupling to proton is not usually observed except in the smallest and most symmetric molecules such as BH4- (fig. 3).
Fig. 3. 10B-NMR spectrum of BH4- in D2O
Properties of 10B
| Property | Value |
|---|---|
| Spin | 3 |
| Natural abundance | 19.9% |
| Chemical shift range | 210 ppm, from -120 to 90 |
| Frequency ratio (Ξ) | 10.743658% |
| Reference compound | 15% BF3.OEt2 in CDCl3 |
| Linewidth of reference | 9 Hz |
| T1 of reference | 0.5 s |
| Receptivity rel. to 1H at natural abundance | 3.96 × 10-3 |
| Receptivity rel. to 1H when enriched | 0.0199 |
| Receptivity rel. to 13C at natural abundance | 23.2 |
| Receptivity rel. to 13C when enriched | 117 |
| Linewidth parameter | 14 fm4 |
11Boron
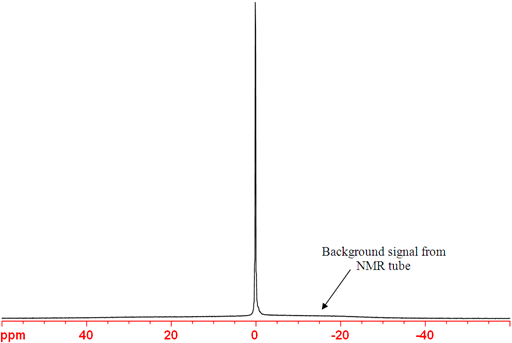
11B-NMR (fig. 4) is more sensitive and yields sharper signals than 10B and is therefore usually the boron nuclide of choice. The coupling between boron and fluorine contain positive and negative components that cancel each other out so that a singlet is observed for BF3 (Fig. 4). A broad signal in the spectrum arising from the boron in the NMR tube is apparent in the spectrum below.
Fig. 4. 11B-NMR spectrum of BF3.OEt2 in CDCl3
Coupling to proton is not usually observed except in the smallest and most symmetric molecules such as BH4- (fig. 5).
Fig. 5. 11B-NMR spectrum of BH4- in D2O
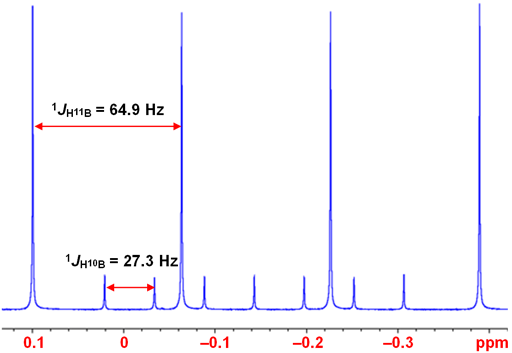
The proton spectrum of BH4- displays couplings to 11B (quartet) and 10B (septet) (fig. 6).
Fig. 6. 1H-NMR spectrum of BH4- in D2O showing coupling to 10B and 11B
Properties of 11B
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 80.1% |
| Chemical shift range | 210 ppm, from -120 to 90 |
| Frequency ratio (Ξ) | 32.083974% |
| Reference compound | 15% BF3.OEt2 in CDCl3 |
| Linewidth of reference | 5 Hz |
| T1 of reference | 0.5 s |
| Receptivity rel. to 1H at natural abundance | 0.165 |
| Receptivity rel. to 1H when enriched | 0.206 |
| Receptivity rel. to 13C at natural abundance | 777 |
| Receptivity rel. to 13C when enriched | 970 |
| Linewidth parameter | 22 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, BF3.OEt2 is toxic, corrosive and reacts violently with water and BF4- is toxic: wear protective gloves and work in a hood. Many other boron compounds are toxic.