Current activity
Major earlier activities
|
Current activity
A. Molecularly doped metals
A new family of materials, organically doped metals (organics@metal), was introduced in the past decade in our labs.
These hybrid materials combine two very different chemical families – the metals, and the very large group of organic molecules.
As a result, these materials not only combine properties of both families,
but lead to synergetic effects in applications ranging from catalysis to bio-activity and much more as detailed below.
The general approach for the preparation of the metallic composites involves a room temperature,
one-pot, reduction of the metal cation, in the presence of the desired organic molecule, with carefully selected reducing agents.
Several techniques were developed for the entrapment of the organic component within the metals: 1. Homogenous syntheses, using water-soluble metal-cation reductants. 2. Homogenous syntheses, using reducing solvents (DMF). 3. Heterogeneous methods in which the reductant is a sacrificial metal. 4. Electrochemical entrapment in which the cation reduction is effected by the application of an electrical potential difference. 5. Using zero-valent metals, either as their complexes or in amalgams. These processes, when initiated, generate metallic nanocrystals which aggregate and accumulate around the organic molecules, thus leading to their entrapment. The composites contain the organic component in the range of 0.2% to 10% w/w, a sufficient amount to induce unique properties in the resulting material. Typically, water soluble dopants are not extracted by water after the doping is completed (but are easily washed away in an adsorption control experiment). A wide array of composites were synthesized by incorporating small molecules, polymers or enzymes in silver, copper, gold, cobalt, palladium and iron and in silver-gold, copper-palladium and copper-platinum and silver-mercury alloys. In all of these combinations the dopant molecules remain intact during the entrapment process and the entrapped molecules are available for chemical reaction. Depending on the preparation conditions and on the environment, the dopant can either be tightly held in the matrix, or the metal can behave as a matrix for controlled release. Furthermore, the entrapped molecules can affect metal properties such as the conductivity and corrosion resistance; they can induce new properties such as acidity and chirality; they provides improved catalytic metals; and they allowed the construction of an electrochemical cell based on same-metal electrodes one of which is doped. Synergism has been often observed, for instance between an entrapped catalyst and a catalytic entrapping metal; and between an entrapped antibacterial molecule and an entrapping antibacterial metal (silver, copper). Detailed studies employing SEM, XRD, adsorption/desorption measurement, density measurements, TGA and more, have provided a tentative picture of physical caging inside partially closed pores, the walls of which are the faces of nano crystallites. Ample evidence exists that this 3D process is completely different than 2D adsorption; only the former has led to the wide scope of useful applications discovered so far. B. The Measurement of Symmetry and Chirality: Concepts and applications
We have been developing the notion that structural chemistry is too rich to be described with the
coarse binary language of either being or not being symmetric or chiral. We have proposed that it agrees with chemical,
biochemical and physical intuition to ask questions such as: “What is the symmetry content of a molecule
(the structure of which cannot be described in exact symmetry terms?)“; or, “given a set of chiral molecules,
by how much do they differ from each other in their chirality content?”; and so on.
Addressing the need to answer this type of structural questions, which are common to many domains of chemistry,
we showed that the problem of how to quantify symmetry and achirality is solvable.
Towards this goal we have developed the Continuous Symmetry Measure (CSM),
the resulting Continuous Chirality Measure (CCM) and, together with Prof. S. Alvarez and his colleagues from Barcelona,
also the Continuous Shape measure.
In essence, the measures quantify the distance of a given structure from the desired ideal symmetry,
or from achirality, or from a reference shape.
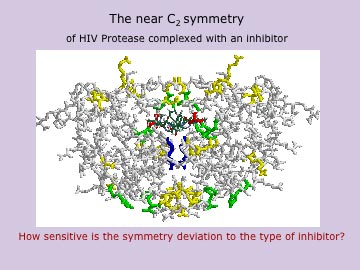
This approach proved useful in a number of symmetry/chirality related issues all across chemistry. Examples include: The application of the symmetry measure as an order parameter in small clusters; the use of continuous symmetry and chirality in the elucidation of new enzymatic structure/activity correlations; the quantitative approach to chiral properties of numerous molecules and clusters; the quantitative treatment of enantiomerization pathways; the analysis of the correlation between the degree of centrosymmetricity and hyperpolarizability; the quantitative evaluation of the relation between parity-violation energy differences and the degree of chirality; the quantitative and conceptual analyses of the chirality of large random objects; the analysis of concerted reactions in terms of quantitative imperfect symmetry; the analysis of the chirality of crystals; the elucidation of the relation between the degree of chirality of a catalyst and the enantiomeric excess of the product; the correlation between pressure and the symmetry of the building blocks of materials; the correlation between the degree of symmetry and the allowedness of spectral transitions; the correlation between the degree of symmetry and the energy content of tetracoordinated molecules; the quantitative evaluation of temperature and pressure effects on the degree of chirality of quartz; the detailed analysis of the origin of symmetry deviations in nearly-symmetric protein clusters; and much more. Three major findings emerged from these studies:
C. Sol-Gel Hybrid Materials: Entrapment of Organic and Bioorganic Molecules
A key materials technology has been gradually developed since the early 80’s.
It allows the incorporation of organic and bioorganic molecules within ceramic materials.
Traditionally, this has been impossible, because of the very high temperatures employed in glasses and ceramic technology.
Now, the properties of ceramic materials can be altered to create a very wide range of previously unknown materials,
by doping of glasses and ceramics with practically any of the millions of organic, polymeric and bioorganic molecules known today.
That development was made possible by the utilization of a room temperature procedure
for the preparation of glasses and other ceramics known as the "sol-gel" process.
It involves a polymerization reaction (rather than the classical melting technology)
in the presence of the host molecule and results in a porous material which has the chemical composition of glass,
looks like glass (it is transparent), and behaves like glass. As a consequence of the wide scope of this hybrid materials technology,
it touches upon many domains of modern needs.
Following are some of the demonstrated applications of the new technology accumulated both at The Hebrew University,
and by many research groups worldwide. These applications can be divided into four major areas:
In the past three decades sol-gel science has completed the full road from basic science to market products and to the establishments of companies based on that technology. Future holds for us many more applications and many more products. D. Astrochemistry
The major revolution in modern astronomy recognizing the universe as teeming with exoplanets, the discovery of liquid water in solar moons,
and the continuing focus on Mars exploration, all accelerate the re-evaluation of potential biomarkers for extraterrestrial life.
Based on life on planet Earth which relies heavily on chiral molecules and especially on homochiral families,
the detection of molecules with these structural properties appears in all road-maps as prime indicators of extraterrestrial life.
This topic of research analyzes the strengths, bounds and potential weaknesses of relying on chirality and on homochirality as biomarkers,
along with recommendations of how to practically use it. Some of the main issues include: what is the extent to which chirality
can be expected to be a universal feature of life; is detection of chirality enough or do we need also to detect homochirality;
how justified is it to view life on Earth as purely homochiral; what are the weaknesses of the need to invent an arbitrary label of handedness
(needed to define homochirality) and what are the pitfalls that emerge from these weaknesses; what stands behind a detected specific value
of enantiomeric excess and what affects its values as we consider old, extinct life, just emerging embryonic life, or extant but rare life;
how can one quantify the degree of homochirality; and, what are relevant experimental approached for detecting chirality on-ground and from distance?
Major earlier activities
A. Fractal Geometry in Chemistry and Physics
Under this topic, various investigations of the description of complex geometries of surfaces
and materials have been undertaken, with special emphasize on the study of the effects that
such complex geometries have on adsorption, reaction and catalysis. Specifically, the studies,
which have included computer simulations, experimental studies and theoretical formulations of
these problems, dealt with accessibility analyses in surface adsorption and derivatizations,
with dynamics of electronic energy transfer, with reactivity of surfaces, with reactivity of
catalysts, with x-ray and neutron scattering from disordered systems, with polymer structures
and polymer adsorption on irregular surfaces, with kinetics and thermodynamics of adsorption
on irregular surfaces, with diffusion limited reactions, with the kinetics of dissolution of
drug particles, and more.
We addressed the very limited scaling range which is typical of most of the experimentally derived fractals, and of the justification of using this term to describe them, and concluded that despite the limited range, in the vast majority of experimental studies, the derivation of an exponent which allows the condensation of a wealth of data on the effects of complex geometries, justifies this empirical approach. Interestingly we found that randomness in its most elementary and pure forms, generates apparent fractal structures over 1-2 decades. B. Chemically Driven Hydrodynamic Instabilities
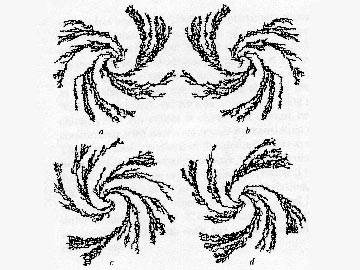
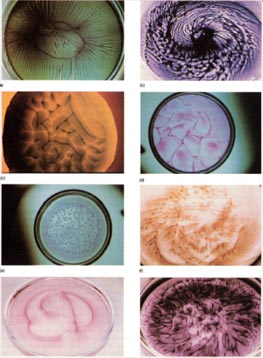
Both experimental and theoretical work has been conducted to understand the origin of pattern
formation at liquid interfaces due to reactions (photochemical, gas/liquid, membranes).
The phenomenon we revealed in the early Eighties is perhaps the widest known in the area
of spatial chemical patterning, from the point of view of the number of different reactions
in which it was found. Detailed analysis of the dynamics of the evolution of the
supramolecular patterns was conducted.
|
|||||||||||||||||||