COSY
1H-1H COSY (COrrelated SpectroscopY) is a useful method for determining which signals arise from neighboring protons (usually up to four bonds). Correlations appear when there is spin-spin coupling between protons, but where there is no coupling, no correlation is expected to appear.
This method is very useful when the multiplets overlap or when is extensive second order coupling complicates the 1D spectrum.
There are many variants on the COSY pulse sequence. The most popular one in our laboratory is the gradient enhanced double quantum coherence (DQF-COSY) version. The ratio of gradient strengths is usually set to two to yield all COSY signals but may be set to three to yield only those correlations involving three protons, e.g., CH-CH2. We use the gradient enhanced DQF-COSY pulse sequence shown in fig. 1.
Fig. 1. Pulse sequence for gradient DQF-COSY

The COSY spectrum as shown in fig. 2 for ethylbenzene (fig. 3) contains a diagonal and cross peaks (signals that are not on the diagonal and correspond to other signals on the same horizontal and vertical projections). The cross peaks indicate couplings between two mutliplets up to three, or occasionally four, bonds away. The diagonal consists of the 1D spectrum with single peaks suppressed. The most apparent cross-peak in the spectrum is between H1' and H2' at 2.65 and 1.24 ppm. A much weaker four-bond correlation (see the figure below) appears between H1' and H2 at 2.65 and 7.20 ppm. All the desired signals are antiphase. Half the multiplet is positive and half negative. In addition, artifacts (undesired signals) appear in the spectrum as vertical streaks (interference and f1 noise) and along the inverted 'V' (fig. 4) whose tip is on the top axis of the sepctrum. These artifacts are rarely in phase with the desired signals and appear in specific locations.
Fig. 2. 2D COSY spectrum of ethylbenzene
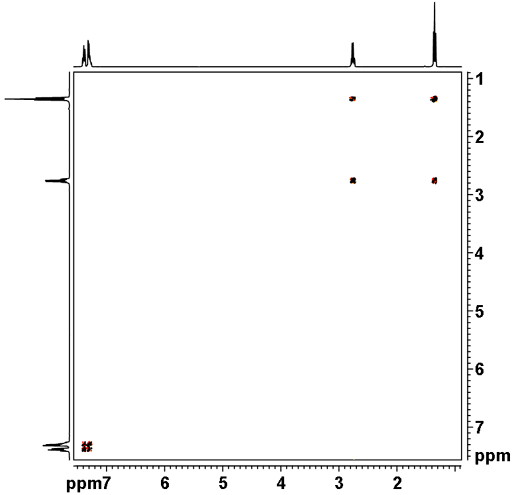
Fig. 3. Structure of ethylbenzene
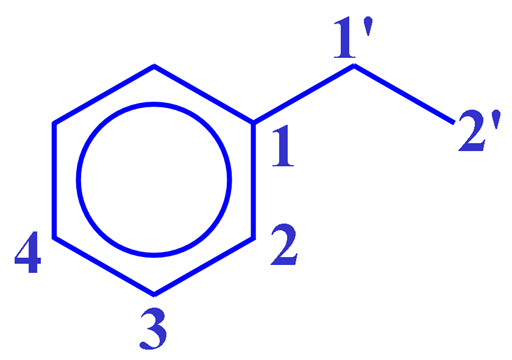
Fig. 4. Artifacts in the COSY spectrum of ethylbenzene

For example, in 12,14-ditbutylbenzo[g]chrysene (fig. 5), only a partial analysis of the regular 1H-NMR spectrum is possible. COSY (fig. 6) provides extra information about the connectivity. No correlations (cross-peaks) are seen to the tbutyls because they are too many bonds away from the ring system.
Fig. 5. Structure of 12,14-ditbutylbenzo[g]chrysene
![12,14-ditbutylbenzo[g]chrysene](cosy_files/dtbbgc.gif)
Fig. 6. Artifacts in the COSY spectrum of ethylbenzene
![COSY of 12,14-ditbutylbenzo[g]chrysene](cosy_files/cosydtbbgc.gif)
The aromatic region of the spectrum (fig. 7) shows three bond correlations strongest. These can be used to determine which protons are neighbors. For example the proton at 8.17 ppm is next to the proton at 7.34 ppm, a fact that could not be easily determined from the 1D spectrum.
Fig. 7. Aromaric region of the 2D COSY spectrum of 12,14-ditbutylbenzo[g]chrysene showing mostly three-bond correlations (a four-bond correlation between H10 and H11 is also visible)
![Aromatic region of COSY of 12,14-ditbutylbenzo[g]chrysene](cosy_files/aromaticdtbbgc.gif)
Four-bond and five-bond correlations are apparent when plotted to lower contours (fig. 8). These separate the spectrum into four groups of protons in a manner that is much clearer than the 1D spectrum.
Fig. 8. Aromaric region of the 2D COSY spectrum of 12,14-ditbutylbenzo[g]chrysene showing three, four and five-bond correlations
![Aromatic region of COSY of 12,14-ditbutylbenzo[g]chrysene](cosy_files/aromaticdtbbgc2.gif)
Using horizontal and vertical lines, it is possible to separate each group and follow its connectivity (fig. 9). The blue group of four protons is connected in the order 8.62 ppm to 7.55 to 7.59 to 8.56, the green group of four protons in the order 8.54 to 7.34 to 7.44 to 8.17 and the red group or two protons, that correspond to H9 and 10 because they are the only group of two protons expected to have a three-bond coupling constant (8.9 Hz), are at 7.76 and 8.32 ppm. The yellow group of two protons correspond to H11 and 13 because the coupling constant is small (1.9 Hz) and consistent with a four bond correlation.
Fig. 9. Aromaric region of the 2D COSY spectrum of 12,14-ditbutylbenzo[g]chrysene showing connectivity and separation into four color-coded proton groups
![Aromatic region of COSY of 12,14-ditbutylbenzo[g]chrysene in color](cosy_files/coloraromatic.gif)
The multiplet structures in COSY are anti-phase for active couplings which leads to different patterns that for pure phase. A pure singlet (A) will not appear in a DQF-COSY spectrum because it is purely single quantum and a double-quantum filter is applied. This is true for deuterated solvent signals and for some protiated solvents such as water. Advantage of this is often taken for solvent suppression. A simple doublet appears in anti-phase. Many other combinations exist. The more common ones are listed in the table below and a few examples are shown.
Table 1. multiplicities often seen in COSY spectra compared with their pure phase counterparts.a
| Multiplicity | Pure phase | Anti-phase |
|---|---|---|
| A | 1 | 0 |
| AX | 1 1 | 1 -1 |
| AX2 | 1 2 1 | 1 0 -1 |
| AXY | 1 1 1 1 | 1 1 -1 -1 |
| AYX | 1 1 1 1 | 1 -1 1 -1 |
| AX3 | 1 3 3 1 | 1 1 -1 -1 |
| AX2Y | 1 2 1 1 2 1 | 1 0 -1 1 0 -1 |
| AY2X | 1 2 1 1 2 1 | 1 2 1 -1 -2 -1 |
| AXY2 | 1 1 2 2 1 1 | 1 -1 2 -2 1 -1 |
| AYX2 | 1 1 2 2 1 1 | 1 -1 0 0 1 -1 |
| AYX2, JAY=2JAX | 1 2 2 2 1 | 1 0 0 0 -1 |
| AXYZ | 1 1 1 1 1 1 1 1 | 1 1 1 1 -1 -1 -1 -1 |
| AYXZ | 1 1 1 1 1 1 1 1 | 1 1 -1 -1 1 1 -1 -1 |
| AYZX | 1 1 1 1 1 1 1 1 | 1 -1 1 -1 1 -1 1 -1 |
| AXYZ, JAX=JAY+JAZ | 1 1 1 2 1 1 1 | 1 1 1 0 -1 -1 -1 |
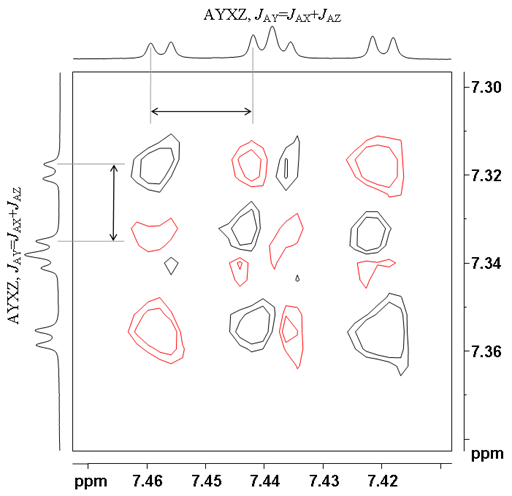
| AXYZ, JAY=JAX+JAZ | 1 1 1 2 1 1 1 | 1 1 -1 0 1 -1 -1 |
| AXYZ, JAZ=JAX+JAY | 1 1 1 2 1 1 1 | 1 -1 1 0 -1 1 -1 |
| AX6 | 1 6 15 20 15 6 1 | 1 2 1 0 -1 -2 -1 |
aThe active coupling is from A to X. The coupling constant decreases from left to right, e.g., for AXYZ, JAX > JAY > JAZ.
Figs. 10-13 show expansions of COSY multiplets. The red contours are negative and the black ones are positive. The 1D projections are only representations (in practice the sum of the projection is zero).
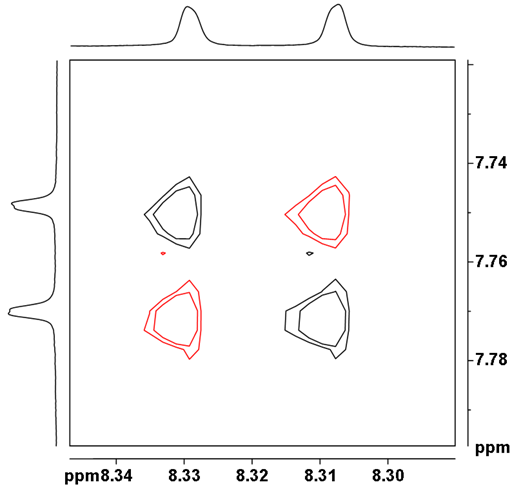
Fig. 10. Anti-phase AX correlation between doublets H9 and 10 of 12,14-ditbutylbenzo[g]chrysene
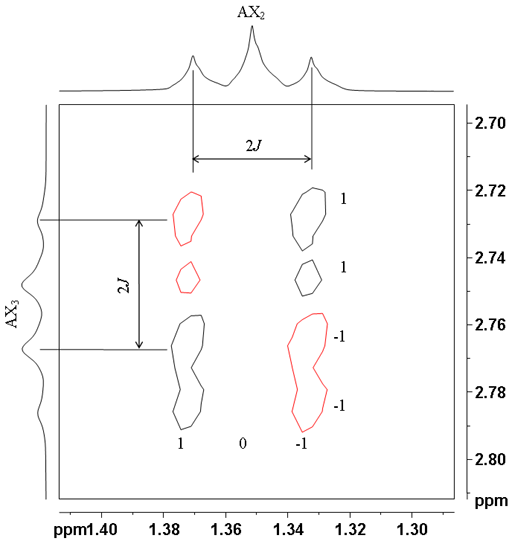
Higher multiplicities in which all the couplings are active yield the patterns shown in table 1. For example the correlation between the CH3 and CH2 protons in ethylbenzene yield a 1 0 1 pattern in one direction and a 1 1 -1 – 1 pattern in the other direction (fig. 11).
Fig. 11. Anti-phase A2X3 correlation between the ethyl protons of ethylbenzene
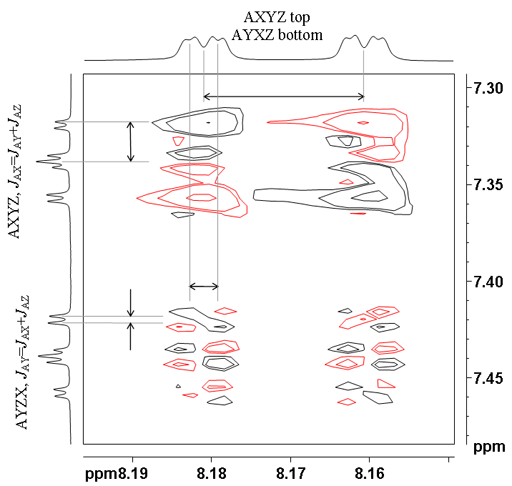
When there are more than two multiplets coupled, then only one coupling is active in each cross-peak. This can be used to determine which coupling constant relates to which correlation, something that may not be obvious from the 1D spectrum. In figs. 12 and 13 for ditbutylbenzo[g]chrysene, the active couplings are labeled. The top cross-peak between the protons at 7.34 and 8.17 ppm shows the largest active coupling of 8.1 Hz. The coupling pattern in the vertical (f1) direction is 1 1 1 0 -1 -1 -1 and in the horizontal (f2) direction it is 1 1 1 1 -1 -1 -1 -1. The multiplet below it has the smallest active coupling of 1.4 Hz. The f1 coupling pattern is 1 -1 1 0 -1 1 -1 and the f2 coupling pattern is 1 1 -1 -1 1 1 -1 -1. The bottom multiplet displays an active coupling of 7.0 Hz and the coupling pattern in both directions is 1 1 -1 0 1 -1 -1.
Figs. 12, 13. Comparison of three AXYZ correlations showing different active couplings (from the COSY of 12,14-ditbutylbenzo[g]chrysene)