(207Pb) Lead NMR
Use our NMR service that provides 207Pb NMR and many other NMR techniques.
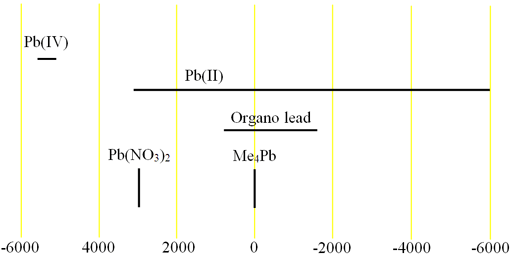
Lead (Pb) has one medium sensitivity NMR spin-½ nucleus, 207Pb (fig. 1) that yields narrow signals over an extremely wide chemical shift range (fig. 2). In liquid-state NMR spectroscpy, Lead NMR is used for studying organo lead compounds. In solid state NMR, the chemical shift of Pb(NO3)2 is used as an NMR thermometer.
Fig. 1. 207Pb-NMR spectrum of Pb(NO3)2 in D2O
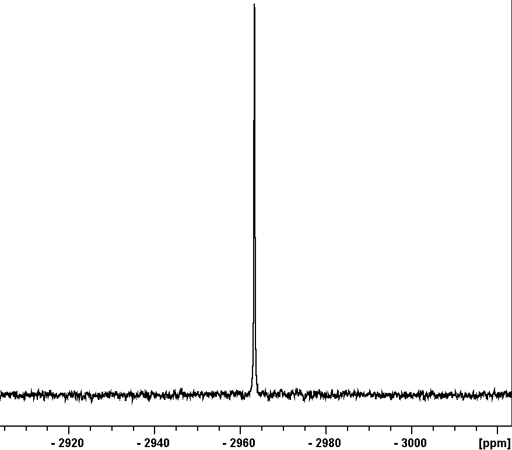
Fig. 2. Chemical shift ranges for lead NMR
Lead shows couplings with other nuclei, 1H of approximately 2500 Hz and 13C up to 16600 Hz.
Properties of 207Pb
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 22.1% |
| Chemical shift range | 11500 ppm, from -5500 to 6000 |
| Frequency ratio (Ξ) | 20.920599% |
| Reference compound | Me4Pb + 5% C6D6 |
| Linewidth of reference | ~1 Hz |
| T1 of reference | ~2 s |
| Receptivity rel. to 1H at natural abundance | 2.00 × 10-3 |
| Receptivity rel. to 1H when enriched | 9.06 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 2.61 |
| Receptivity rel. to 13C when enriched | 11.8 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, lead is very toxic: wear protective clothing.
References
- J. E. H. Buston, T. D. W. Claridge, R. G. Compton, M. G. Moloney, "207Pb NMR chemical shifts of aryllead tricarboxylates" Magn. Reson. Chem. 36, 140-144 (1998).
- V. Sharma, S. Agarwal, R. Bohra, V. K. Jain, "Triethyl-germanium, -tin and -lead complexes derived from internally functionalised oximes" J. Chem. Res. 273-275 (2004).
- H. C. Marsmann, F. Uhlig, Frank, "Further advances in germanium, tin and lead NMR" Ed. Z. Rappoport, "Chemistry of Organic Germanium, Tin and Lead Compounds" 2, 399-459 (2002).
- B. Wrackmeyer, "Application of 207Pb NMR parameters" Ann. Rep. NMR Spectrosc. 47, 1-37, (2002).