(Hg) Mercury NMR
Use our NMR service that provides 199Hg NMR and many other NMR techniques.
Mercury (Hg) has two NMR active nuclei, 199Hg and 201Hg. 199Hg is a low sensitivity spin-½ nucleus that yields sharp signals over a very wide chemical shift range. 201Hg a quadrupolar low sensitivity nucleus that yields signals too broad to be observed with a high-resolution NMR spectrometer even for small molecules such as dimethyl mercury. Therefore, 199Hg is the mercury nucleus of choice. 199Hg-NMR is used for the study of mercury compounds, their structure, dynamics and conformation. It is also used for biological binding studies using its relaxation effects.
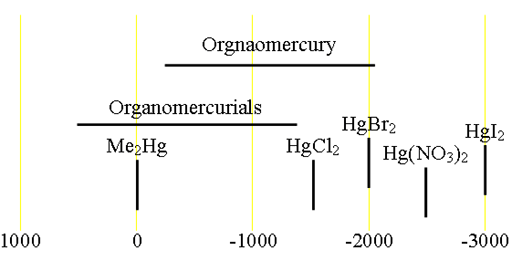
Each type of mercury compound has its characteristic chemical shift range (fig. 1).
Fig. 1. Chemical shift ranges for mercury NMR
199Mercury NMR
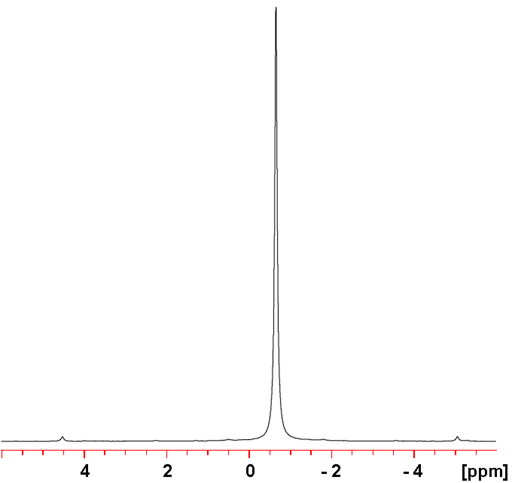
199Hg yields sharp signals (fig. 2) and is more sensitive than 201Hg. Therefore 199Hg is the mercury nucleus of choice.
Fig. 2. 199Hg-NMR, proton decoupled, of Me2Hg (neat)
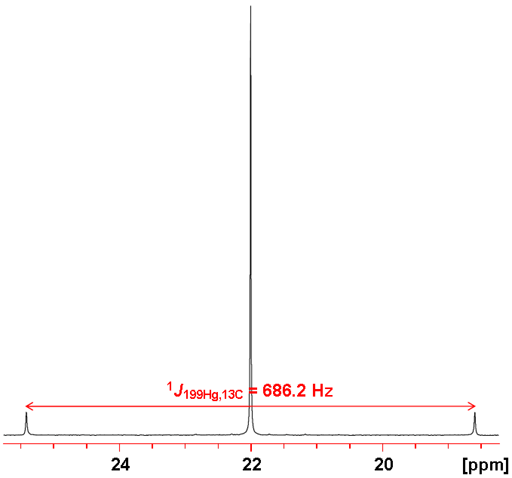
199Hg couples with many nuclei. Two-bond 1H-199Hg couplings are between 100 and 270 Hz (figs. 3 and 4). One-bond couplings to 13C are between 600 and 3000 Hz (figs. 5 and 6), two-bond from 70 to 130 Hz and three-bond 100 to 220 Hz.
Fig. 3. 199Hg-NMR of Me2Hg (neat) showing proton coupling
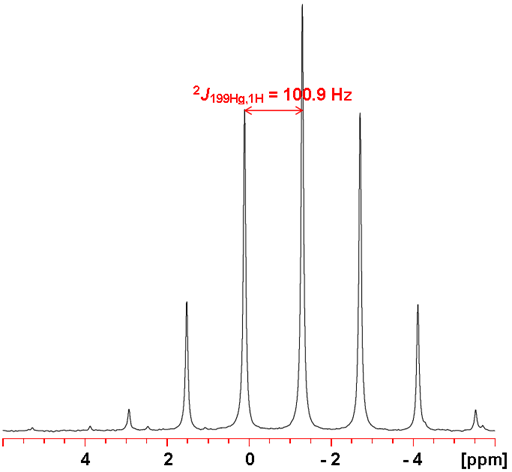
Fig. 4. 1H-NMR of Me2Hg (neat) showing coupling to 199Hg

Fig. 5. 199Hg-NMR of Me2Hg (neat) showing coupling to 13C

Fig. 6. 13C-NMR of Me2Hg (neat) showing coupling to 199Hg
Properties of 199Hg
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 16.87% |
| Chemical shift range | 3500 ppm, from -3000 to 500 |
| Frequency ratio (Ξ) | 17.910822% |
| Reference compound | Me2Hg (neat) |
| Linewidth of reference | 2.6 Hz |
| T1 of reference | 0.5 s |
| Receptivity rel. to 1H at natural abundance | 1.00 × 10-3 |
| Receptivity rel. to 1H when enriched | 5.93 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 5.89 |
| Receptivity rel. to 13C when enriched | 34.9 |
201Mercury NMR
201Hg is a quadrupolar nucleus that yields signals too broad cannot be observed on a high resolution NMR spectrometer even for small molecules such as Me2Hg. 201Hg is also less sensitive than 199Hg. Therefore, 201Hg is not the mercury nucleus of choice for NMR. Because it is unobservable, we have no useful experience of 201Hg-NMR in our laboratory.
Properties of 201Hg
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 13.18% |
| Chemical shift range | 3500 ppm, from -3000 to 500 |
| Frequency ratio (Ξ) | 6.611583% |
| Reference compound | Me2Hg (neat) |
| Linewidth of reference | >40000 Hz |
| T1 of reference | <0.00002 s |
| Receptivity rel. to 1H at natural abundance | 1.97 × 10-4 |
| Receptivity rel. to 1H when enriched | 1.49 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 1.16 |
| Receptivity rel. to 13C when enriched | 8.80 |
| Linewidth parameter | 2000 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, mercury salts are very toxic: wear protective gloves and work in a hood. Dimethyl mercury and other organomercuries are very toxic (LD50 Me2Hg, 0.1 mL!): wear protective clothing and work in a hood. Latex gloves do not provide protection. Highly resistant laminate gloves (SilverShield or 4H) should be worn under a pair of long-cuffed, unsupported neoprene, nitrile, or similar heavy-duty gloves. One drop can kill.
References
- V. S. Petrosyan, and O. A. Reutov, "Study of the structure and complexation of organic and inorganic derivatives of metals by means of NMR spectroscopy of heavy nuclei", Pure Appl. Chem., 37, 147-59 (1974).
- M. Borzo and G. E. Maciel, "Mercury-199 chemical shifts of organomercury compounds by Fourier transform NMR", J. Magn. Reson., 19, 279-282 (1975).
- N. K. Wilson, R. D. Zehr and P. D. Ellis, "Carbon-13 nuclear magnetic resonance. Carbon-13 chemical shifts and carbon-13-mercury-199 coupling constants for some organomercury compounds", J. Magn. Reson., 21, 437-443 (1976).
- J. L. Sudmeier and T. G. Perkins, " Studies of single 199HgII ion resonances in the active site of human carbonic anhydrase B by Fourier transform nuclear magnetic resonance", J. Am. Chem. Soc., 99, 7732-7733 (1977).
- A. J. Canty, A. Marker, P. Barron and P. C. Healy, "A mercury-199 NMR spectroscopic study of two- and three-coordinate methylmercury(II) complexes, [MeHgL]NO3", J. Organometal. Chem., 144, 371-379 (1978).
- Y. A. Strelenko, Y. G. Bundel, F. H. Kasumov, V. I. Rozenberg, O. A. Reutov and Y. A. Ustynyuk, "σ,π,-Conjugation and mercury-199 shielding constants in benzyl derivatives of mercury", J. Organometal. Chem., 159, 131-135 (1978).
- M. J. Albright and J. P. Oliver, "Studies on main group metal-transition-metal bonded compounds. 7. A mercury-199 NMR study of some Group VI transition-metal mercury compounds", J. Organometal. Chem., 172, 99-107 (1979).
- R. Colton and D. Dakternieks, "Phosphorus-31 and mercury-199 NMR studies on mercury (II) halide-tributylphosphine complexes", Aust. J. Chem., 33, 955-963 (1980).
- R. Meyer, L. Gorrichon-Guigon and P. Maroni, "Carbon-13 and mercury-199 NMR study on oxobromo- and dioxomercuric compounds", J. Organometal. Chem., 188, 11-24 (1980).
- M. F. Roberts, D. A. Vidusek and G. Bodenhausen, "Adducts of ethylmercury phosphate with amino acids studied by indirect detection of mercury-199 NMR", FEBS Lett., 117, 311-314 (1980).
- P. R. Wells and D. W. Hawker, "Mercury-199 NMR chemical shifts in substituted diphenylmercury and phenylmercuric chloride", Org. Magn. Reson., 17, 26-27 (1981).
- M. J. Albright, T. F. Schaaf, A. K. Hovland and J. P. Oliver, "Metal-silicon bonded compounds. XVIII. A mercury-199 FT NMR study of some silylmercury derivatives and selected organomercury compounds", J. Organometal. Chem., 259, 37-50 (1983).
- A. M. Bond, R. Colton, M. L. Dillon, J. E. Moir and D. R. Page, "Investigation of exchange and redox reactions of mercury dithiocarbamate complexes by electrochemical techniques at mercury electrodes, mercury-199 nuclear magnetic resonance spectrometry and mass spectrometry", Inorg. Chem., 23, 2883-2889 (1984).
- A. R. Norris and R. Kumar, "Mercury-199 NMR correlations in methylmercury(II) complexes of nucleic acid constituents and their analogs", Inorg. Chim. Acta, 93, L63-L65 (1984).
- P. A. W. Dean and R. S. Srivastava, "A multinuclear (proton, phosphorus-31, mercury-199) nuclear magnetic resonance study of some complexes of mercury (II) with ditertiary phosphines", Can. J. Chem., 63, 2829-2839 (1985).
- K. E. Rowland and R. D. Thomas, "Carbon-13 and mercury-199 NMR data for methyl-substituted diarylmercury compounds", Magn. Reson. Chem., 23, 916-919 (1985).
- G. B. Deacon, M. J. O'Connor and G. N. Stretton, "Organomercury compounds. XXVIII. The synthesis and mercury-199 NMR spectra of some unsymmetrically dimercurated arenes", Aust. J. Chem., 39, 953-962 (1986).
- B. F. Abrahams, M. Corbett, D. Dakternieks, R. W. Gable, B. F. Hoskins, E. R. T. Tiekink and G. Winter, "NMR studies of phosphine adducts of mercury and cadmium xanthates and halo xanthates. Crystal and molecular structures of Cd(S2COPr-iso)2PPh3, Hg(S2COPr-iso)2PPh3 and Hg(S2COPr-iso)2P(c-C6H11)3 (c-C6H11 = cyclohexyl)", Aust. J. Chem., 39, 1993-2001 (1986).
- L. V. Pankratov, I. M. Penyagina, L. N. Zakharov, M. N. Bochkarev, G. A. Razuvaev, Y. K. Grishin, Y. A. Ustynyuk and Y. T. Struchkov, "Reactivity of germylmercurate complexes", J. Organometal. Chem., 335, 313-322 (1987).
- M. M. Kubicki, J. Y. Le Gall, R. Pichon, J. Y. Salaun, M. Cano and J. A. Campo, "95Mo and 199Hg NMR studies on complexes containing molybdenum-mercury bonds and substituted cyclopentadienyl ligands: [(C5H5-nRn)(CO)3Mo]xHgX2-x (R = Me, n = 0, 1, 4, 5; R = Ph, n = 4; X = Cl, Br, I; x = 1, 2)", J. Organometal. Chem., 348, 349-356 (1988).
- M. Delnomdedieu, D. Georgescauld, A. Boudou and E. J. Dufourc, "Mercury-199 NMR. A tool to follow chemical speciation of mercury compounds", Bull. Magn. Reson., 11, 420 (1989).
- M. Delnomdedieu, A. Boudou, D. Georgescauld and E. J. Dufourc, "Specific interactions of mercury chloride with membranes and other ligands as revealed by mercury – NMR", Chem. Bio. Interact., 81, 243-269 (1992).
- M. Cano, J. A. Campo, J. Y. Le Gall, R. Pichon, J. Y. Salaun and M. M. Kubicki, "Molybdenum- mercury bond. NMR (mercury-199, phosphorus-31, proton) and IR study on [(C5H5)(CO)2LMoHgZ] [L = P(4-XC6H4)3 (X = F, Cl, Me, OMe), P(CH2CH3)3, P(CH2CH2CN)3; Z = Cl, I, (C5H5)(CO)2LMo] complexes", Inorg. Chim. Acta, 193, 207-212 (1992).
- L. Yang, J. Chen, X. Lei, Y. Wu and M. Song, "Substituent effect on mercury-199 chemical shifts in some bisarylmercurials and aryl(2-benzothiazolylthio)mercurials", Chem. Res. Chin. Univ., 8, 81-83 (1992).
- X. Yang, Z. Zheng, C. B. Knobler and M. F. Hawthorne, "Anti-crown" chemistry: synthesis of [9]mercuracarborand-3 and the crystal structure of its acetonitrile complexes", J. Am. Chem. Soc., 115, 193-195 (1993).
- Y. K. Grishin, V. V. Orlov, G. A. Artamkina and Y. A. Ustynyuk, "Mercury-199 nuclear magnetic shielding constants of benzyl mercury derivatives", Zh. Organich. Khim., 30, 1601-1607 (1994).
- Y. J. Wu, S. Q. Huo, H. Z. Yuan and Y. H. Liu, "Secondary interaction and n-π conjugation in ferrocenylimine derivatives of mercury as probed by Hg-199 NMR", Main Group Chem., 1, 253-256 (1996).
- A. Berra, M. L. Di Vona, B. Floris and S. Licoccia, "199Hg NMR: a tool for direct detection of the products from acetoxymercuration of alkynes", Appl. Organometal. Chem., 14, 565-569 (2000).
- F. S. H. Vieco, N. M. Hiramatsu, E. Tedeschi, D. B. Rezende and I. P. A. Campos, "The γ-cis effect in the mercury-199 NMR spectroscopy of substituted vinylmercury halides", J. Chem. Res., Syn., 25-27 (2002).