(Ba) Barium NMR
Use our NMR service that provides Ba NMR and many other NMR techniques.
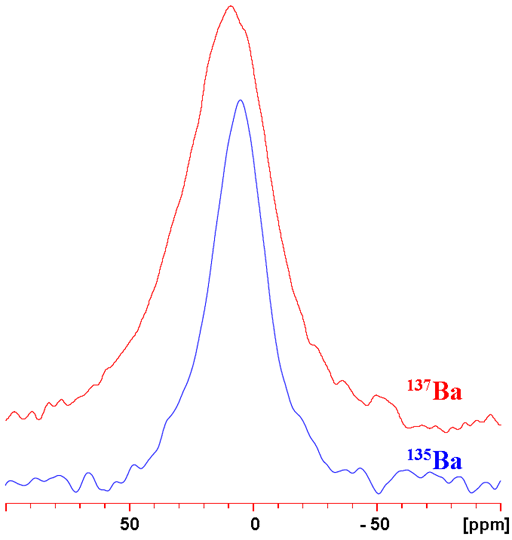
Barium (Ba) has two low sensitivity NMR active nuclei, 135Ba and 137Ba. 137Ba is more sensitive but yields broader signals than 135Ba (fig. 1). 137Ba is the preferred barium nucleus as resolution has yet to be shown to be important because no barium NMR spectrum containing more than one peak has been reported. Both nuclei are quadrupolar and yield very broad signals even in symmetric environments. Barium has a narrow chemical shift range, narrower than its line-width. As a result barium NMR is hardly used and when used, it is for determining its aqueous binding properties from its relaxation rate.
Fig. 1. Comparison of 135Ba and 137Ba NMR for BaCl2 (1 M) in D2O
There is hardly any information about barium chemical shifts (fig. 2).
Fig. 2. Chemical shift ranges for barium NMR
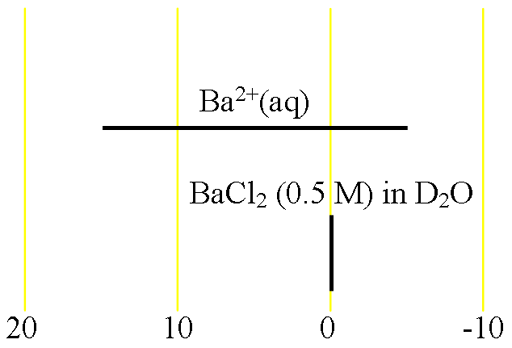
135Barium NMR
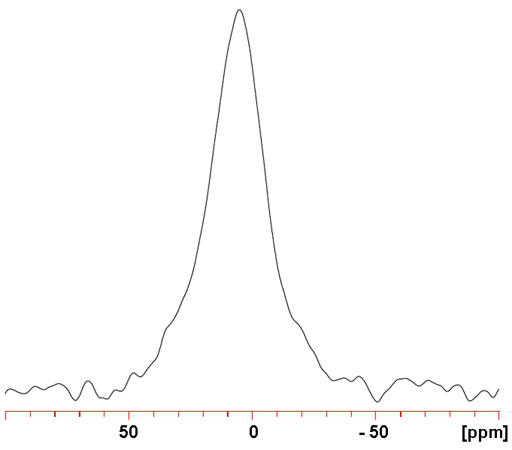
135Ba (fig. 3) yields signals that are less sensitive and broad than 137Ba. However, because no spectrum showing more than one barium signal has been reported, resolution is not important so 137Ba NMR is the preferred barium nucleus.
Fig. 3. 135Ba NMR for BaCl2 (1 M) in D2O
Properties of 135Ba
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 6.592% |
| Chemical shift range | 20 ppm, from -15 to 5 |
| Frequency ratio (Ξ) | 9.934457% |
| Reference compound | 0.5 M BaCl2 in D2O |
| Linewidth of reference | 707 Hz |
| T1 of reference | 0.00022 s |
| Receptivity rel. to 1H at natural abundance | 3.30 × 10-4 |
| Receptivity rel. to 1H when enriched | 5.01 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 1.93 |
| Receptivity rel. to 13C when enriched | 29.3 |
| Linewidth parameter | 340 fm4 |
137Barium NMR
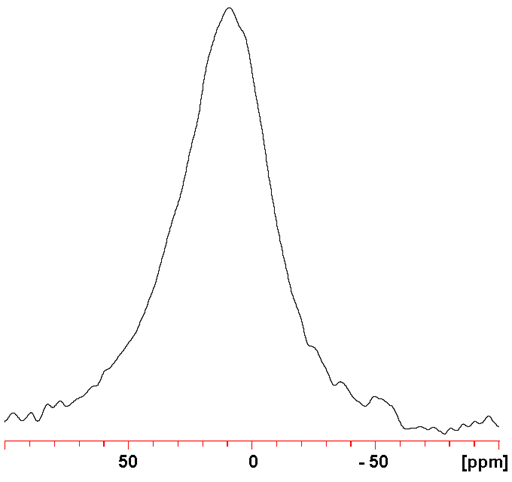
137Ba (fig. 4) yields signals that are more sensitive and broader than 135Ba. However, because no spectrum showing more than one barium signal has been reported, resolution is not important so 137Ba NMR is the preferred barium nucleus.
Fig. 4. 137Ba NMR for BaCl2 (1 M) in D2O
Properties of 137Ba
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 11.232% |
| Chemical shift range | 20 ppm, from -15 to 5 |
| Frequency ratio (Ξ) | 11.112928% |
| Reference compound | 0.5 M BaCl2 in D2O |
| Linewidth of reference | 1425 Hz |
| T1 of reference | 0.00006 s |
| Receptivity rel. to 1H at natural abundance | 7.87 × 10-4 |
| Receptivity rel. to 1H when enriched | 7.01 × 10-3 |
| Receptivity rel. to 13C at natural abundance | 4.62 |
| Receptivity rel. to 13C when enriched | 41.1 |
| Linewidth parameter | 800 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, soluble barium salts including BaCl2 are toxic (LD50 1 g) insoluble salts are less toxic: wear gloves.
References
- O. Lutz and H. Oehler, "Barium-135 and -137 Fourier transform NMR and NQR studies", Z. Phys. A, 288, 11-15 (1978).
- H. Krueger, O. Lutz and H. Oehler, H. "Nuclear magnetic moments and ratios of quadrupole moments barium-135 and -137 and lanthanum-138 and -139 by NMR spectroscopy", Phys. Lett. A, 62A, 131-2 (1977).
- V. I. Chizhik, "NMR relaxation and microstructure of aqueous electrolyte solutions", Mol. Phys., 90, 653-659 (1997).