(83Kr) Krypton NMR
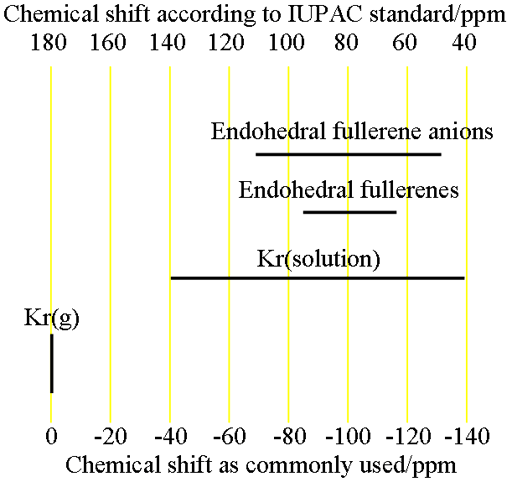
Krypton has one naturally occurring NMR active nucleus, 83Kr (fig. 1). It has a low sensitivity and a spin of 9/2 and is therefore quadrupolar although it is always found in highly symmetrical environments so its signals are narrow and spread out over a wide chemical shift range (fig. 2). The chemistry of krypton is limited to that of endoherdral fullerenes and the unstable KrF2 molecule although the only NMR spectra reported are of the unreacted gas, either free or in solution. We have no experience of 83Kr in our laboratory although we could proved such a service if provided with a suitable sample.
Fig. 1. 83Kr NMR spectrum of krypton gas
(endohedral fullerenes have never been measured and their chemical shifts are as theoretically predicted)
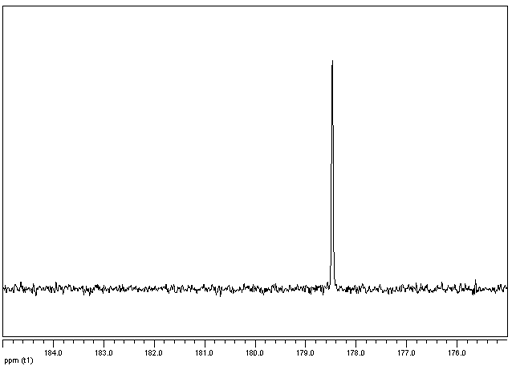
Spectrum courtesy of W. Makulski and K. Jackowski
Fig. 2. Chemical shift ranges for krypton NMR
Properties of 83Kr
| Property | Value |
|---|---|
| Spin | 9/2 |
| Natural abundance | 11.49% |
| Chemical shift range | 140 ppm, from -140 to 0 (IUPAC 40 to 180) |
| Frequency ratio (Ξ) | 3.848287% (IUPAC 3.847600%) |
| Reference compound | Kr gas |
| Linewidth of reference | 0.6 Hz |
| T1 of reference | 1 s |
| Receptivity rel. to 1H at natural abundance | 1.09 × 10-4 |
| Receptivity rel. to 1H when enriched | 9.49 × 10-4 |
| Receptivity rel. to 13C at natural abundance | 0.642 |
| Receptivity rel. to 13C when enriched | 5.59 |
| Linewidth parameter | 50 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, krypton displaces oxygen in the air and is therefore an asphyxiant.
References
- Mazitov R. K., Enikeev K. M., Ilyasov A. V., "Magnetic Resonance and Relaxation of Nuclei of Atomic Krypton in Liquid Solutions", Z. Phys. Chem., 155, 56-68 (1987).