(57Fe) Iron NMR
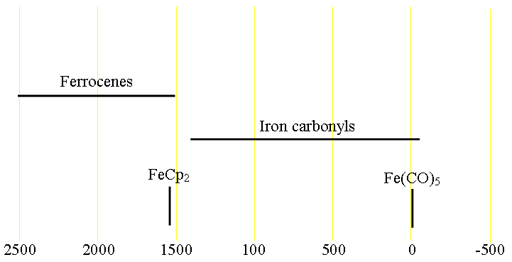
57Iron (57Fe) is a very low sensitivity spin ½ nucleus with a low (2.119%) natural abundance that yields narrow lines over a very wide chemical shift range. Its extremely low sensitivity and low resonant frequency, outside the range of standard NMR probes, limits its use. Only very pure oxidation states of iron(0) and Iron(II) (fig. 1) are sufficiently diamagnetic to yield a high resolution NMR signal. Other oxidation states are paramagnetic. There is little information available about chemical shifts (fig. 2).
Fig. 1. 57Fe-NMR spectrum of ferrocene (2M) in CDCl3. The signal is broadened by proton coupling.
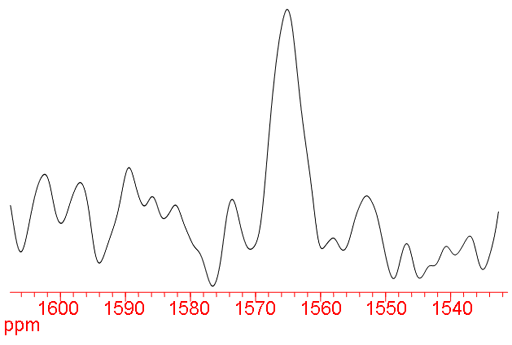
Fig. 2. Chemical shift ranges for iron NMR
Properties of 57Fe
| Property | Value |
|---|---|
| Spin | 1/2 |
| Natural abundance | 2.119% |
| Chemical shift range | 2600 ppm, from -100 to 2500 |
| Frequency ratio (Ξ) | 3.237778% |
| Reference compound | Fe(CO)5 |
| Linewidth of reference | ~0.5 Hz |
| T1 of reference | ~3 s |
| Receptivity rel. to 1H at natural abundance | 7.24 × 10-7 |
| Receptivity rel. to 1H when enriched | 3.41 × 10-5 |
| Receptivity rel. to 13C at natural abundance | 4.25× 10-3 |
| Receptivity rel. to 13C when enriched | 0.201 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, ferrocenes are toxic and iron pentacarbonyl is extremely toxic, work in a hood and use appropriate gloves and protective clothing.