(Cu) Copper NMR
Use our NMR service that provides Cu NMR and many other NMR techniques.
Copper (Cu) has two NMR active nuclei, 63Cu and 65Cu with a very wide chemical shift range (fig. 1). Both nuclei are quadrupolar and therefore yield broad signals in small molecules and signals, too broad to be observed with a high-resolution NMR spectrometer, in medium and large molecules. 63Cu is the more sensitive nucleus while 65Cu yields very slightly narrower signals (fig. 2). Usually though 63Cu NMR is preferred.
Fig. 1. Chemical shift ranges for copper NMR
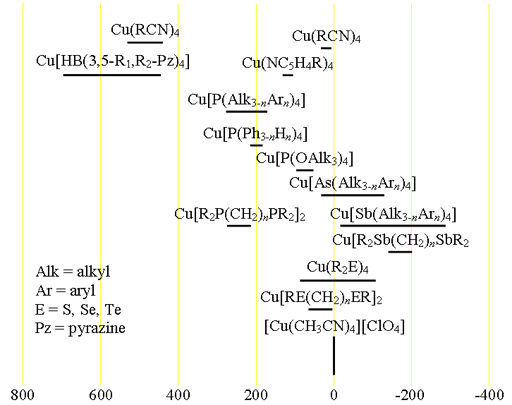
Fig. 2. Comparison of 63Cu and 65Cu for CuCl in CD3CN. 63Cu yields greater sensitivity while 65Cu yields marginally narrower signals.

Each type of copper complex has its characteristic chemical shift range (fig. 1). Copper NMR is only observed for oxidation state (I) because (II) is paramagnetic.
63Copper NMR
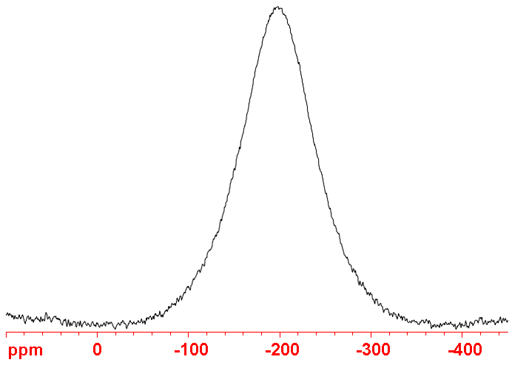
63Cu (fig. 3) is usually the nucleus of choice since it is more sensitive than 65Cu even though it yields marginally broader signals.
Fig. 3. 63Cu-NMR spectrum of CuCl in CD3CN
Properties of 63Cu
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 69.7% |
| Chemical shift range | 1100 ppm, from -300 to 800 |
| Frequency ratio (Ξ) | 26.515473% |
| Reference compound | [Cu(CH3CN)4][ClO4] + 10% C6D6 |
| Linewidth of reference | 400 Hz |
| T1 of reference | 0.0004 s |
| Receptivity rel. to 1H at natural abundance | 0.0650 |
| Receptivity rel. to 1H when enriched | 0.0940 |
| Receptivity rel. to 13C at natural abundance | 382 |
| Receptivity rel. to 13C when enriched | 552 |
| Linewidth parameter | 650 fm4 |
65Copper NMR
65Cu (fig. 4) is less sensitive than 63Cu even though it yields marginally narrower signals. 65Cu would only be the nucleus of choice for isotopic enrichment studies or in the unlikely event that resolution is critical.
Fig. 4. 65Cu-NMR spectrum of CuCl in CD3CN

Properties of 65Cu
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 30.83% |
| Chemical shift range | 1100 ppm, from -300 to 800 |
| Frequency ratio (Ξ) | 28.403693% |
| Reference compound | [Cu(CH3CN)4][ClO4] + 10% C6D6 |
| Linewidth of reference | 400 Hz |
| T1 of reference | 0.0004 s |
| Receptivity rel. to 1H at natural abundance | 0.0354 |
| Receptivity rel. to 1H when enriched | 0.115 |
| Receptivity rel. to 13C at natural abundance | 208 |
| Receptivity rel. to 13C when enriched | 675 |
| Linewidth parameter | 550 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, copper salts are toxic.