(Br) Bromine NMR
Use our NMR service that provides Br NMR and many other NMR techniques.
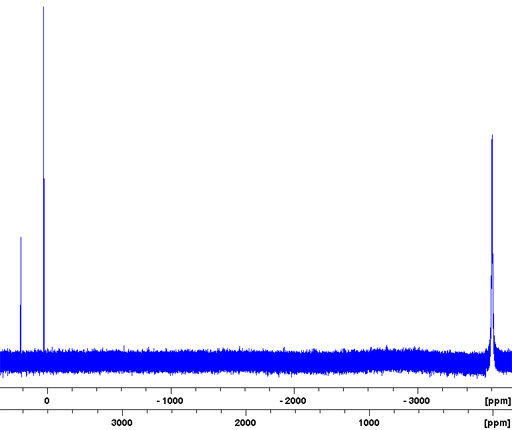
Bromine (Br) has two NMR active nuclei, 79Br and 81Br with a wide chemical shift range (fig. 1). Both nuclei are quadrupolar and therefore yield broad signals as ions in symmetrical environments and signals, too broad to be observed with a high-resolution NMR spectrometer, even in small molecules. As a result bromine NMR is restricted to relaxation studies of the binding of bromide ions. 81Br is the more sensitive nucleus and yields slightly narrower signals than 79Br (fig. 2) so 81Br is the nucleus of choice. However, 79Br has a frequency very similar to 13C (fig. 3) and is often observable using a carbon specific probe. Because of this and because it produces clear quadrupolar spinning sidebands in the solid state (fig. 4), it is widely used in solid state NMR for adjusting the magic angle. There is very little information about bromine chemical shifts (fig. 1).
Fig. 1. Chemical shift ranges for bromine NMR
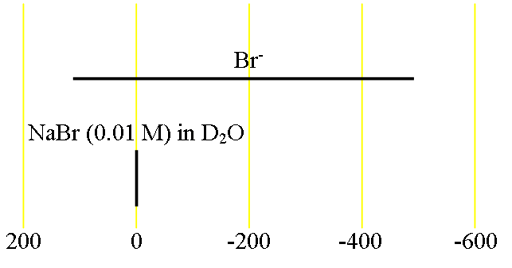
Fig. 2. Comparison of 79Br and 81Br for KBr in D2O. 81Br is the more sensitive nucleus and yields narrower signals.
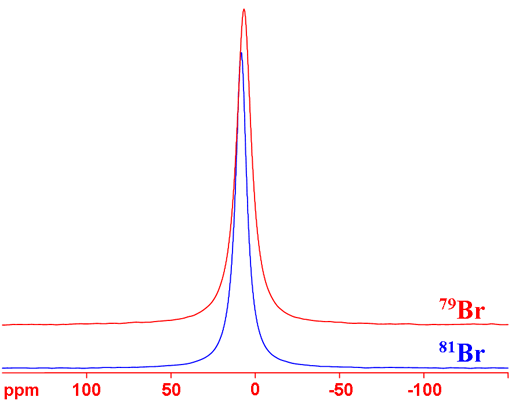
Fig. 3. The signal of 79Br (right) in the same spectrum as the 13C signals (left) for KBr and acetone in D2O. The chemical shift scales are for 13C (top) and 79Br (bottom).
79Bromine NMR
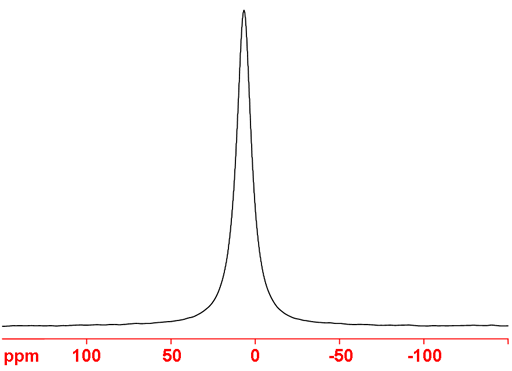
79Br (fig. 5) is less sensitive and yields broader signals than 81Br so it is not usually the nucleus of choice for liquid NMR. However, 79Br has a frequency very similar to 13C (fig. 3) and is often observable using a carbon specific probe. Because of this and because it produces clear quadrupolar spinning sidebands in the solid state (fig. 4), it is widely used in solid state NMR for adjusting the magic angle.
Fig. 4. 79Br-NMR spectrum of solid KBr showing spinning sidebands used for adjusting the magic angle
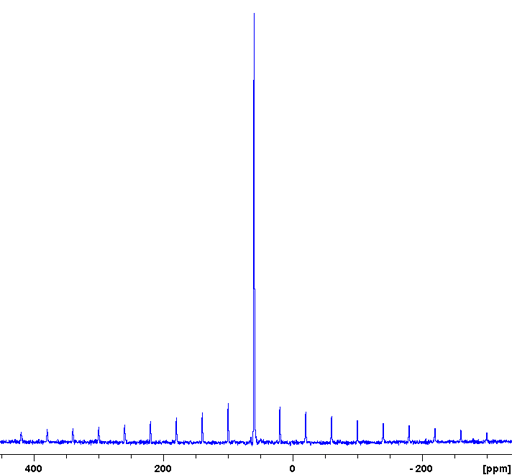
Fig. 5. 79Br-NMR spectrum of KBr in D2O
Properties of 79Br
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 50.69% |
| Chemical shift range | 600 ppm, from -500 to 100 |
| Frequency ratio (Ξ) | 25.053980% |
| Reference compound | NaBr (0.01 M) in D2O |
| Linewidth of reference | 648 Hz |
| T1 of reference | 0.0005 s |
| Receptivity rel. to 1H at natural abundance | 0.0403 |
| Receptivity rel. to 1H when enriched | 0.0795 |
| Receptivity rel. to 13C at natural abundance | 237 |
| Receptivity rel. to 13C when enriched | 468 |
| Linewidth parameter | 1300 fm4 |
81Bromine NMR
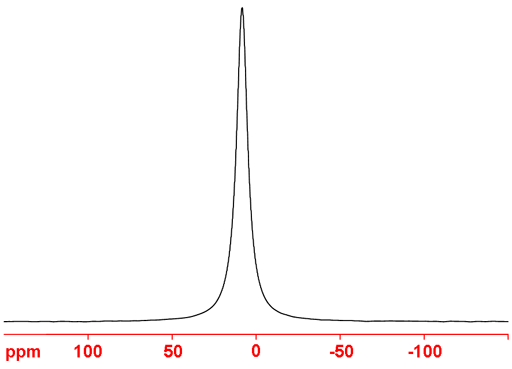
81Br (fig. 6) is the bromine nucleus of choice, at least for liquid-state NMR, as it is more sensitive and yields narrower signals than 79Br.
Fig. 6. 81Br-NMR spectrum of KBr in D2O
Properties of 81Br
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 49.31% |
| Chemical shift range | 600 ppm, from -500 to 100 |
| Frequency ratio (Ξ) | 27.006518% |
| Reference compound | NaBr (0.01 M) in D2O |
| Linewidth of reference | 467 Hz |
| T1 of reference | 0.002 s |
| Receptivity rel. to 1H at natural abundance | 0.0491 |
| Receptivity rel. to 1H when enriched | 0.0996 |
| Receptivity rel. to 13C at natural abundance | 288 |
| Receptivity rel. to 13C when enriched | 584 |
| Linewidth parameter | 920 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, bromide is toxic in large doses. Other oxidation states of bromine are toxic: wear gloves.
References
- G. Lindblom, B. Lindman and L. Mandell "Study of counter-ion binding to reversed micelles by nuclear magnetic quadrupole relaxation of bromine-81" J. Coll. Interface Sci., 34, 262-71, (1970).
- P. Diehl, E. Fluck and R. Kosfeld, Eds., "NMR, Basic Principles and Progress, Vol. 12: Chlorine, Bromine and Iodine NMR. Physico-Chemical and Biological Applications." Springer, Berlin, Ger. (1976).
- T. Drakenberg and S. Forsen, "[NMR of] the halogens - chlorine, bromine, and iodine" NATO ASI Series, Series C: Mathematical and Physical Sciences 103 (Multinucl. Approach NMR Spectrosc.), 405-44 (1983).