(23Na) Sodium NMR
Use our NMR service that provides 23Na NMR and many other NMR techniques.
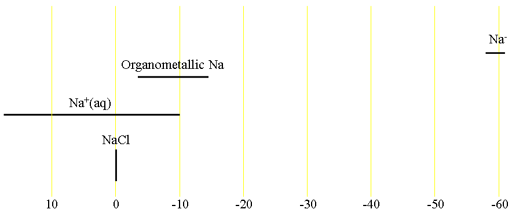
23Sodium (23Na) is a medium sensitivity nucleus that yields slightly broad lines over a moderate chemical shift range. 23Na is a spin 3/2 nucleus and is therefore quadrupolar. As a result, the signal width increases with asymmetry of the environment. Hence, in the spectra in fig. 1, NaCl is narrower than NaOH that is narrower than a PAH salt (disodium 9,10-diphylanthracenediide). The main use of sodium NMR is to determine the presence of sodium or the number of different chemical sites that it occupies. There is little information available about chemical shifts (fig. 2).
Fig. 1. 23Na-NMR spectra of NaOH, NaCl and an organometallic sodium salt showing the variation in line-width
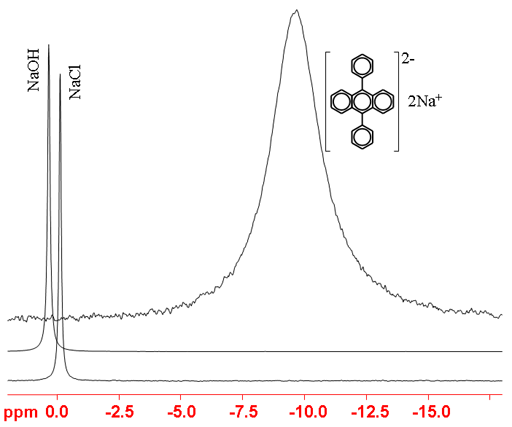
Fig. 2. Chemical shift ranges for 23Na NMR
Properties of 23Na
| Property | Value |
|---|---|
| Spin | 3/2 |
| Natural abundance | 100% |
| Chemical shift range | 72 ppm, from -62 to 10 |
| Frequency ratio (Ξ) | 26.451900% |
| Reference compound | 0.1 M NaCl in D2O |
| Linewidth of reference | 8.2 Hz |
| T1 of reference | 0.1 s |
| Receptivity rel. to 1H at natural abundance | 0.0927 |
| Receptivity rel. to 1H when enriched | 0.0927 |
| Receptivity rel. to 13C at natural abundance | 545 |
| Receptivity rel. to 13C when enriched | 545 |
| Linewidth parameter | 140 fm4 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, sodium salts are non-toxic on account of their sodium under normal circumstances, however, their anions may be toxic.