(3He) Helium NMR
3Helium is a very sensitive nucleus when enriched, yields sharp signals and has a moderate chemical shift range. At the natural abundance of helium extracted from the air it is extremely insensitive. Helium extracted from mineral deposits contains much less 3He than that extracted from the air. However, helium's chemistry is limited to endohedral fullerenes so the use of this NMR nucleus is limited. In addition, its resonance frequency falls outside the range of conventional NMR probes, so special equipment is required.
We are one of only three NMR laboratories in the World equipped to observe high-resolution 3He NMR on a routine basis.
Use our NMR service.
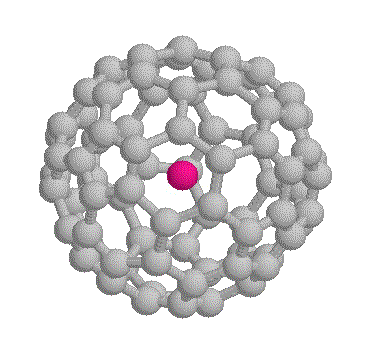
3He tends to have long relaxation times. The gas has a T1 of about 1000 s, 3He@C60 (fig. 1) (the @ symbol indicates that the helium is inside the fullerene cage) has a T1 of about 300 s but by dissolving it in a viscous solvent such as methylnaphthalene and adding oxygen gas, it can be reduced to a more manageable 30 s.
Fig. 1. Molecular diagram of 3He@C60
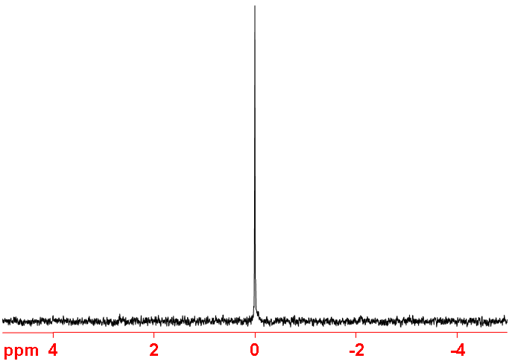
The chemical shift (fig. 2) of 3He is referenced to that of 3He gas (fig. 3) at low pressure (there is no significant change in chemical shift up to 2 atmospheres). Because helium is unreactive and has no intermolecular interactions, its chemical shift is independent on temperature when at reasonable pressures and, although very inconvenient, can be and has been used as a chemical shift standard for other nuclei. Dissolved 3He gas interacts weakly with the solvent and has a chemical shift between -0.8 and 0.2 ppm, the precise chemical shift being related to the polarizability of the solvent.
Fig. 2. 3He chemical shift ranges
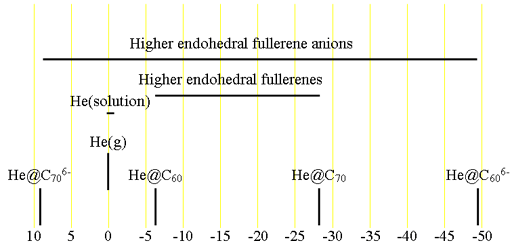
Fig. 3. 3He NMR spectrum of 3He gas
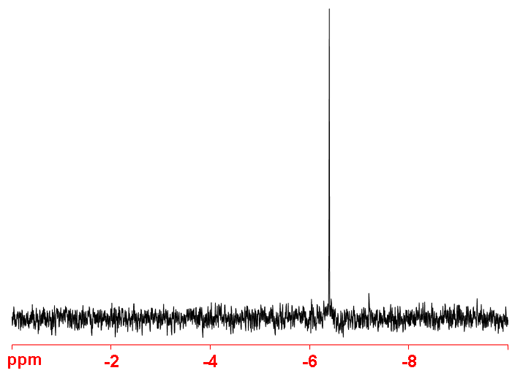
The main chemical use of 3He NMR is the study of endohedral fullerenes. The endohedral atom acts as a measure of aromaticity, the lower the chemical shift, the more aromatic the fullerene. Hence C70 is more aromatic than C60 because the chemical shift of 3He@C70 is -28.0 ppm while that of 3He@C60 is -6.4 ppm (fig. 4).
Fig. 4. 3He NMR spectrum of 3He@C60
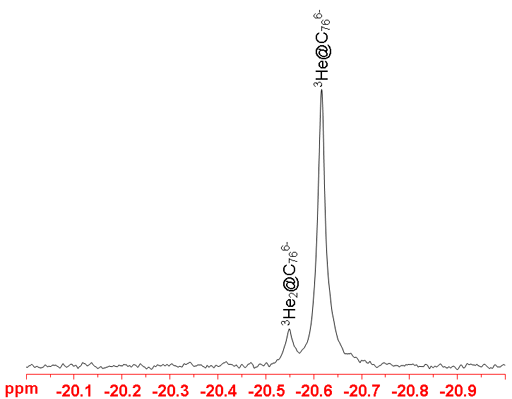
These fullerene spectra can be used to study subtle effects. For example, the addition of a second endohedral helium causes a chemical shift change (fig. 5) that can be used to interpret spacial charge distribution within the fullerene (see T. Sternfeld, R. E. Hoffman, M. Saunders, R. J. Cross, M. S. Syamal and M. Rabinovitz, J. Am. Chem. Soc., 124, 8786 (2002).).
Fig. 5. Spectrum of a mixture of endohedral C76 fullerene anions showing the effect of an extra helium
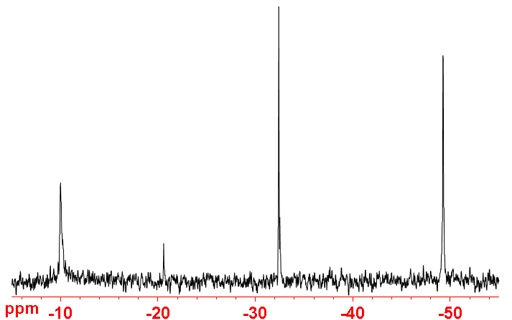
3He NMR is an excellent tool for determining the number of fullerene species present because it is sensitive and only yields one peak per species (fig. 6). By contrast, 13C NMR is insensitive and yields many signals for each of the higher fullerenes (see T. Sternfeld, PhD thesis).
Fig. 6. 3He NMR of a mixture of higher fullerene anions
Properties of 3He
| Property | Value |
|---|---|
| Spin | ½ |
| Natural abundance | 0.000134% |
| Chemical shift range | 58 ppm, from -50 to 8 |
| Frequency ratio (Ξ) | 76.178976% |
| Reference compound | 3He gas = 0 ppm |
| Linewidth of reference | 0.3 Hz |
| T1 of reference | 1000 s |
| Receptivity rel. to 1H at natural abundance | 5.75 × 10-7 |
| Receptivity rel. to 1H when enriched | 0.442 |
| Receptivity rel. to 13C at natural abundance | 3.27 × 10-3 |
| Receptivity rel. to 13C when enriched | 2510 |
Safety note
Some of the materials mentioned here are very dangerous. Ask a qualified chemist for advice before handling them. Qualified chemists should check the relevant safety literature before handling or giving advice about unfamiliar substances. NMR solvents are toxic and most are flammable. Specifically, Helium displaces oxygen in the air and is therefore an asphyxiant.