Oxide nano clusters characterization
TiO2 nanoparticles have usually been produced, in the field of surface science, by oxidising small nanoparticles of pre-deposited Ti with molecular Oxygen gas, at elevated temperatures (annealing at 600K).
In this research, TiO2 nanoparticles have been produced by depositing Ti on amorphous solid water (ASW) at low temperatures and then desorbing the water, by slowly heating the substrate to room-temperature. The Ti adatoms chemically react with the water molecules to form Ti-O bonds in various degrees of oxidation. Full oxidation (to Ti4+) occurs at room-temperature.
This procedure is known as the Reactive Layer Assisted Deposition (RLAD), a technique used for creating compound (mainly oxides) thin films or nanoparticles. Controlling the two main parameters, amount of Ti and the ASW buffer layer thickness, leads to a variety of samples.
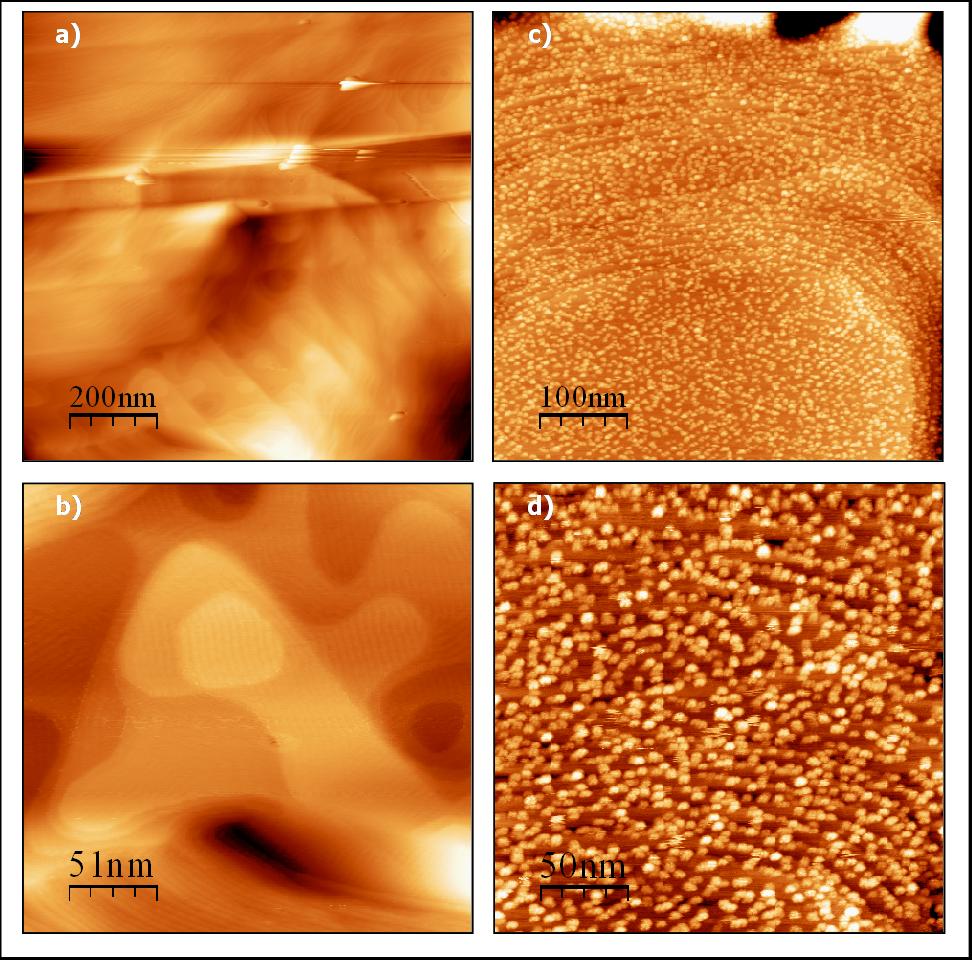
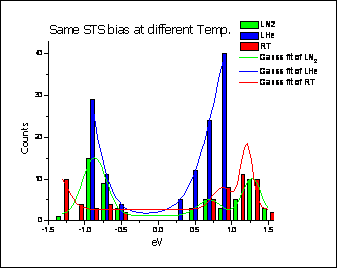
The chemical nature of the TiO2 nanoparticles shell was determined by ex-situ XPS measurements and was found to be of a fully oxidised TiO2 bulk character (Ti4+). The morphology of the nanoparticles was investigated by ex-situ AFM and STM and STS measurements.