Publications
• Substitution of an internal disulfide bridge in human insulin by a diselenide enhances its foldability and stability
Weil-Ktorza, O. Rege, N., Lansky, S., Shalev, D.E., Shoham, G., Weiss, M.A., and Metanis N.* Chem. Eur. J. 2019, Advance Article. DOI: 10.1002/chem.201900892.
Selected for cover of the issue
Featured in the issue's Cover Profile: DOI: 10.1002/chem.201901974
Featured on Israel's National Radio Station Galei Tzahal. Listen here.
• The bacterial extracellular matrix protein TapA is a two‐domain partially disordered protein
Abbasi, R., Mousa, R., Dekel, N., Amartely, H., Danieli, T., Lebendiker, M., Levi-Kalisman, Y., Shalev, D.E., Metanis, N.*, and Chai, L. ChemBioChem 2019, 20(3), 355-359. DOI: 10.1002/cbic.201800634
Selected as Very Important Paper (VIP).
Selected for cover of the issue.
• BPTI folding revisited: switching a disulfide into methylene thioacetal reveals a previously hidden path
Mousa, R., Lansky, S., Shoham, G., and Metanis, N.* Chem. Sci. 2018, 9, 4814-4820. DOI: 10.1039/C8SC01110A
• Young Career Focus: Dr. Norman Metanis (Hebrew University of Jerusalem, Israel)
Synform, 2018, 04, A53-A55, DOI: 10.1055/s-0036-1591462
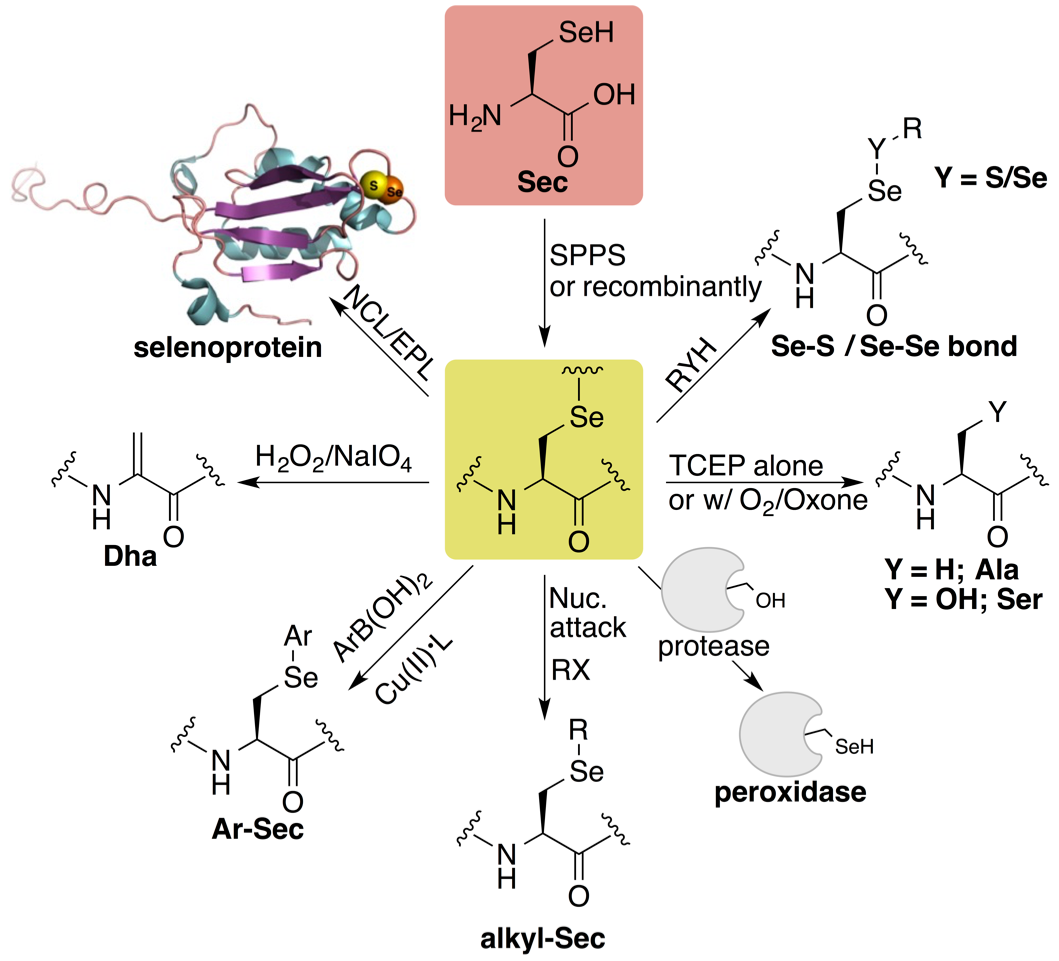
• Selenium and selenocysteine in protein chemistry (minireview)
Mousa, R., Dardashti, R.N., and Metanis, N.* Angew. Chem. Int. Ed. 2017, 56(50), 15818-15827. DOI: 10.1002/anie.201706876
• A combinatorial approach for the synthesis of multi-phosphorylated peptides: new tool for studying phosphorylation patterns in proteins
Samarasimhareddy, M., Mayer, D., Metanis, N., Veprintsev, D., Hurevitch, M., and Friedler, A. bioRxiv, 2017. DOI: 10.1101/196444
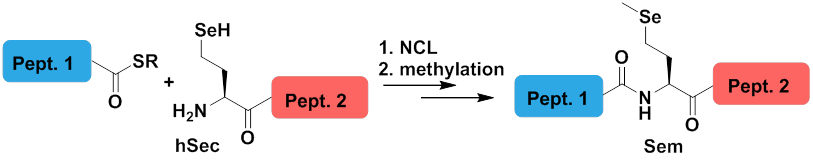
• Revisiting ligation at selenomethionine: insights into native chemical ligation at selenocysteine and homoselenocysteine
Dardashti, R.N. and Metanis, N.* Bioorg. & Med. Chem. 2017, 25(18), 4983-4989. DOI: 10.1016/j.bmc.2017.05.006
• Chemical protein synthesis through selenocysteine chemistry
Mousa, R., Reddy, P.S., and Metanis, N.* Synlett 2017, 28(12), 1389-1393. DOI: 10.1055/s-0036-1588762
• Peptide fibrils as monomer storage of the covalent HIV-1 integrase inhibitor
Chandra, K., Das, P., Metanis, N., Friedler, A., and Reches, M. J. Pept. Sci. 2017, 23(2), 117-121. DOI: 10.1002/psc.2959
• Accessing human selenoproteins through chemical protein synthesis
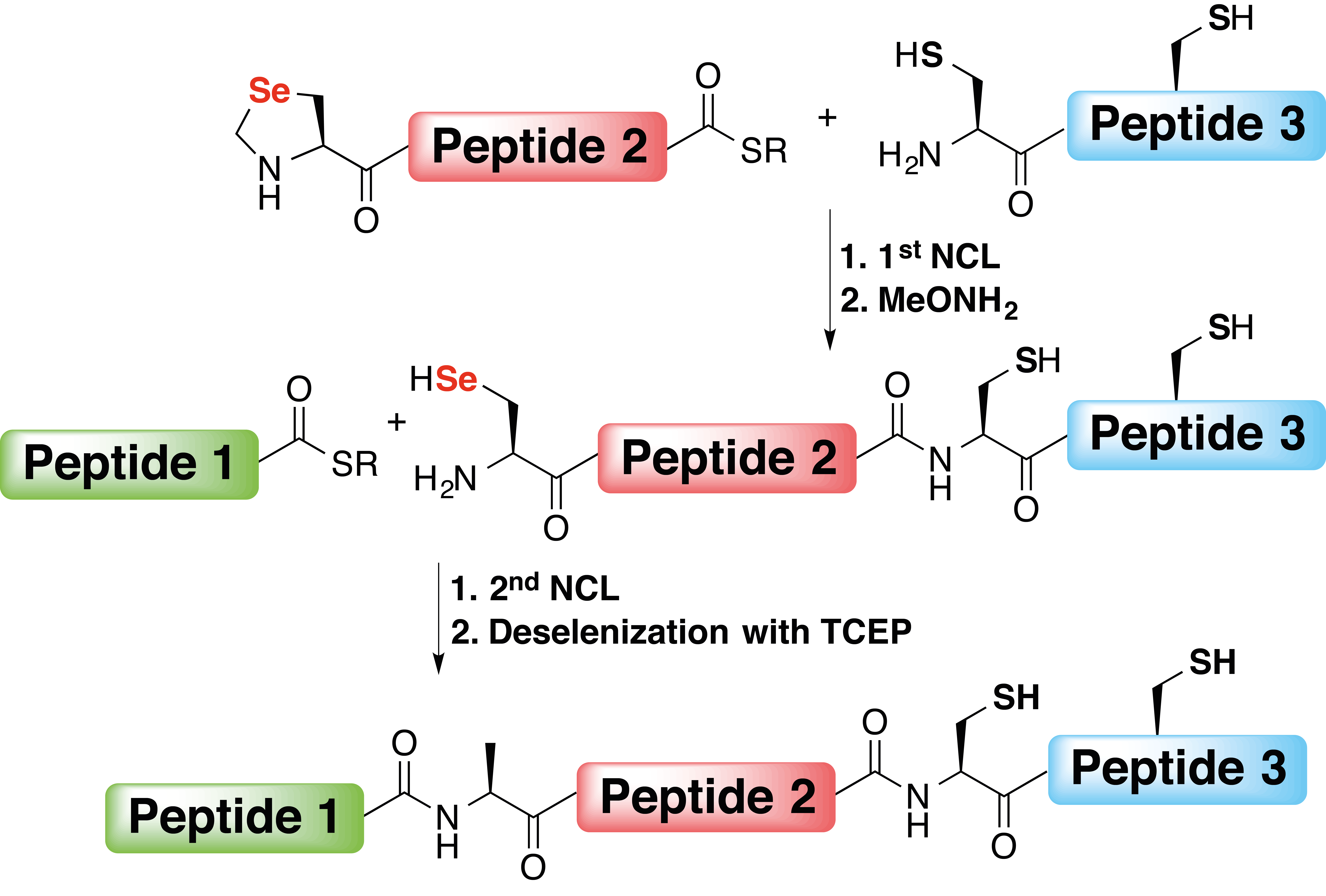
Dery, L., Reddy, P.S., Dery, S., Mousa, R., Ktorza, O., Talhami, A., and Metanis, N.* Chem. Sci. 2017, 8, 1922-1926. DOI: 10.1039/c6sc04123j.
• Selenocysteine containing analogues of Atx-1 based peptides protect cells from copper ion toxicity
Lehman, Y., Shoshan, M.S., Goch, W., Bal, W., Tshuva, E.Y., and Metanis, N.* Org. Biomol. Chem. 2016, 14, 6979-6984. DOI: 10.1039/C6OB00849F
• Covalent inhibition of HIV-1 Integrase by N-succinimidyl peptides
Chandra, K., Das, P., Mamidi, S., Hurevich, M., Iosub-Amir, A., Metanis, N., Reches, M., and Friedler, A. ChemMedChem, 2016, 11, 1987–1994. DOI: 10.1002/cmdc.201600190
Selected for cover of the issue.
• The Chemistry of Selenocysteine in Proteins
Dardashti, R.N., Dery, L., Mousa, R., Dery, S., Reddy, P.S., and Metanis, N.* Selenium: Its Molecular Biology and Role in Human Health, 4th Ed. Springer 2016, 73-83.
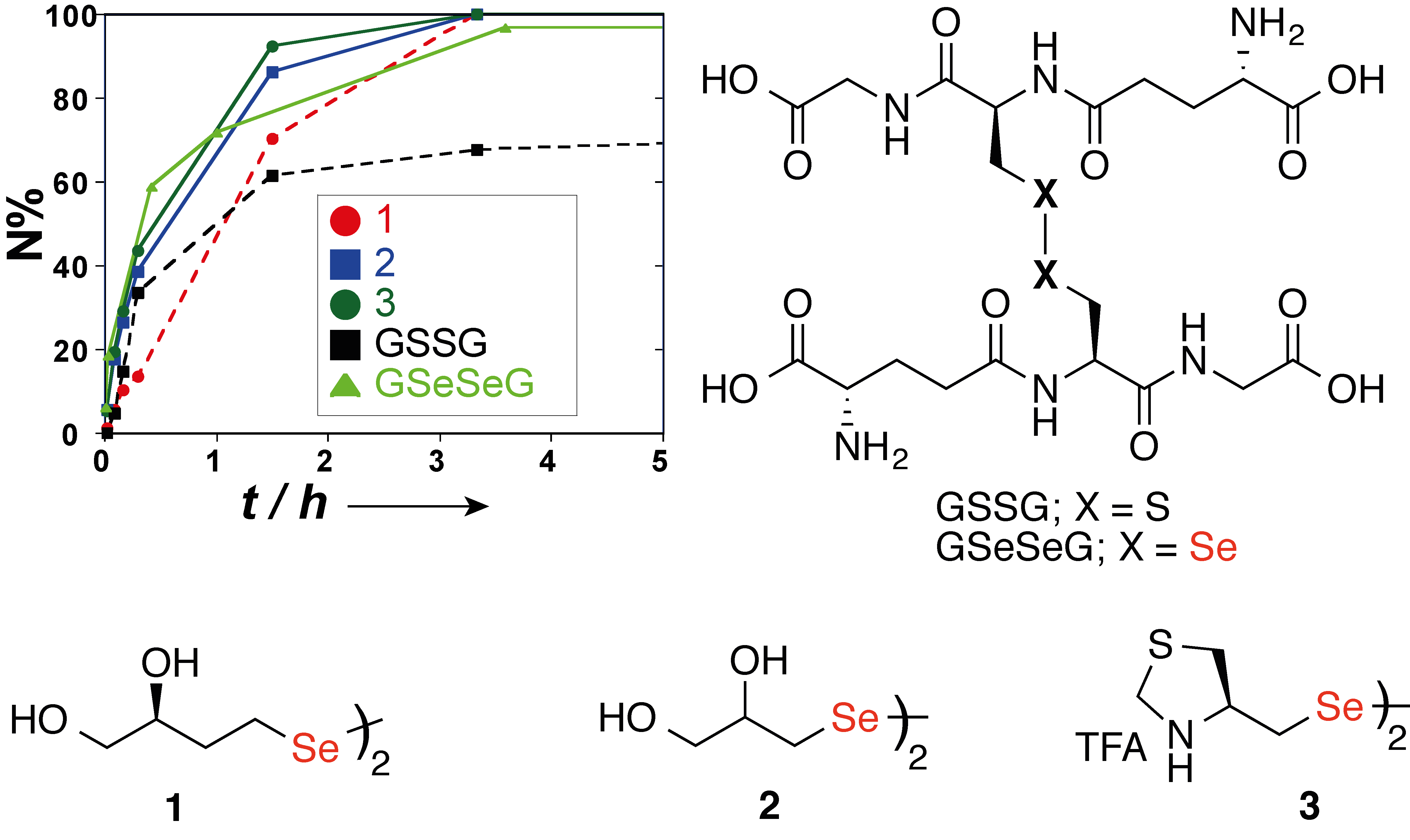
• Small molecule diselenide additives for in vitro oxidative protein folding
Reddy, P.S. and Metanis, N*. Chem.Commun. 2016, 52, 3336-3339. DOI: 10.1039/C5CC10451C
• Chemical synthesis of proteins with non-strategically placed cysteines using selenazolidine and selective deselenization
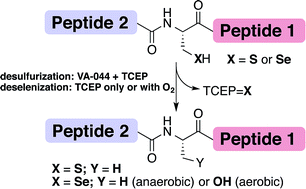
Reddy, P.S., Dery, S., and Metanis, N.* Angew. Chem. Int. Ed. 2016, 55, 992-995. DOI: 10.1002/ange.201509378
• Insights into the deselenization of selenocysteine into alanine and serine
Dery, S., Reddy, P.S., Dery, L., Mousa, R., Dardashti, R.N., and Metanis, N.* Chem. Sci. 2015, 6, 6207-6212. DOI: 10.1039/C5SC02528A
• Harnessing selenocysteine reactivity for oxidative protein folding
Metanis, N.* and Hilvert D. Chem. Sci. 2015, 6, 322-325. DOI: 10.1039/c4sc02379j
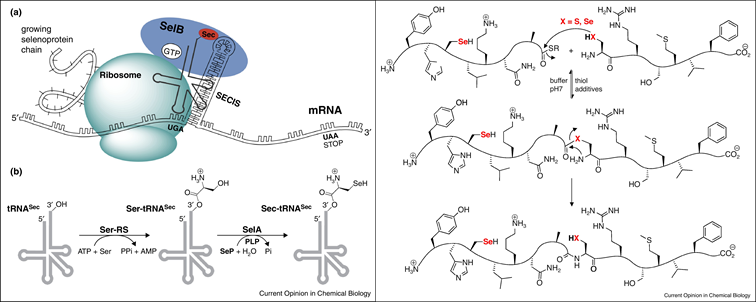
• Natural and synthetic selenoproteins
Metanis, N.* and Hilvert D. Curr. Opin. Chem. Biol. 2014, 22, 27-34. DOI: 10.1016/j.cbpa.2014.09.010
• Chemical Protein Synthesis (CPS) Meeting 2013
Metanis, N.* ChemBioChem 2013, 14, 1381-1384. (Invited Conference Report). DOI: 10.1002/cbic.201300337
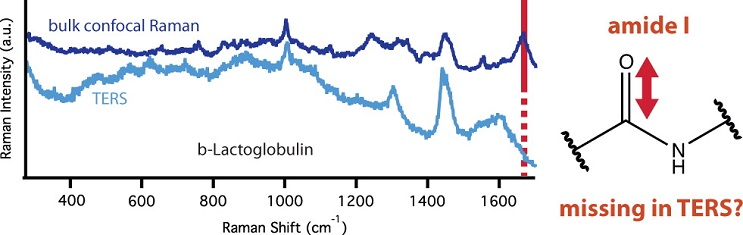
• Missing Amide I mode in gap-mode tip-enhanced Raman spectra of proteins
Blum C.; Schmid T.; Opilik L.; Metanis N.; Widmann S. and Zenobi R., J. Phys. Chem. C 2012, 116, 23061-23066. DOI: dx.doi.org/10.1021/jp306831p
• Strategic use of nonnative diselenide bridges to steer oxidative protein folding
Metanis N. and Hilvert D. Angew. Chem. Int. Ed. 2012, 51, 5585-5588. DOI: 10.1002/anie.201109129
Selected by referees as Very Important Paper (VIP)
Highlighted in Nature Chemistry
Highlighted in ChemBioChem
• The Chemistry of Selenocysteine
Metanis, N.; Beld, J. and Hilvert, D. Patai’s Chemistry of Functional Groups, Ed. Rappoport, Z.; John Wiley & Sons, Ltd., 2011. DOI: 10.1002/9780470682531.pat0582.
• Selenoglutathione-mediated rescue of kinetically trapped intermediate in oxidative protein folding
Metanis, N.; Foletti, C.; Beld, J. and Hilvert, D., Isr. J. Chem. 2011, 51, 953-959. DOI: 10.1002/ijch.201100105
Invited article in special issue
Highlighted in ChemBioChem
• Traceless ligation of cysteine peptides using selective deselenization
Metanis, N.; Keinan, E. and Dawson, P.E., Angew. Chem. Int. Ed. 2010, 49, 7049-7053. DOI: 10.1002/anie.201001900
• A residue outside the active site CXXC motif regulates the catalytic efficiency of Glutaredoxin-3
Shekhter, T.§; Metanis, N.§;Dawson, P.E. and Keinan, E., Mol. BioSyst. 2010, 6, 241-248. §authors contributed equally. DOI: 10.1039/B912753D
• Synthetic seleno-Glutaredoxin 3 analogs are highly reducing oxidoreductases with enhanced catalytic efficiency
Metanis, N.; Keinan, E. and Dawson, P.E. J. Am. Chem. Soc. 2006, 128, 16684-16691. DOI: 10.1021/ja0661414
• A designed synthetic analogue of 4-OT is specific for a non-natural substrate
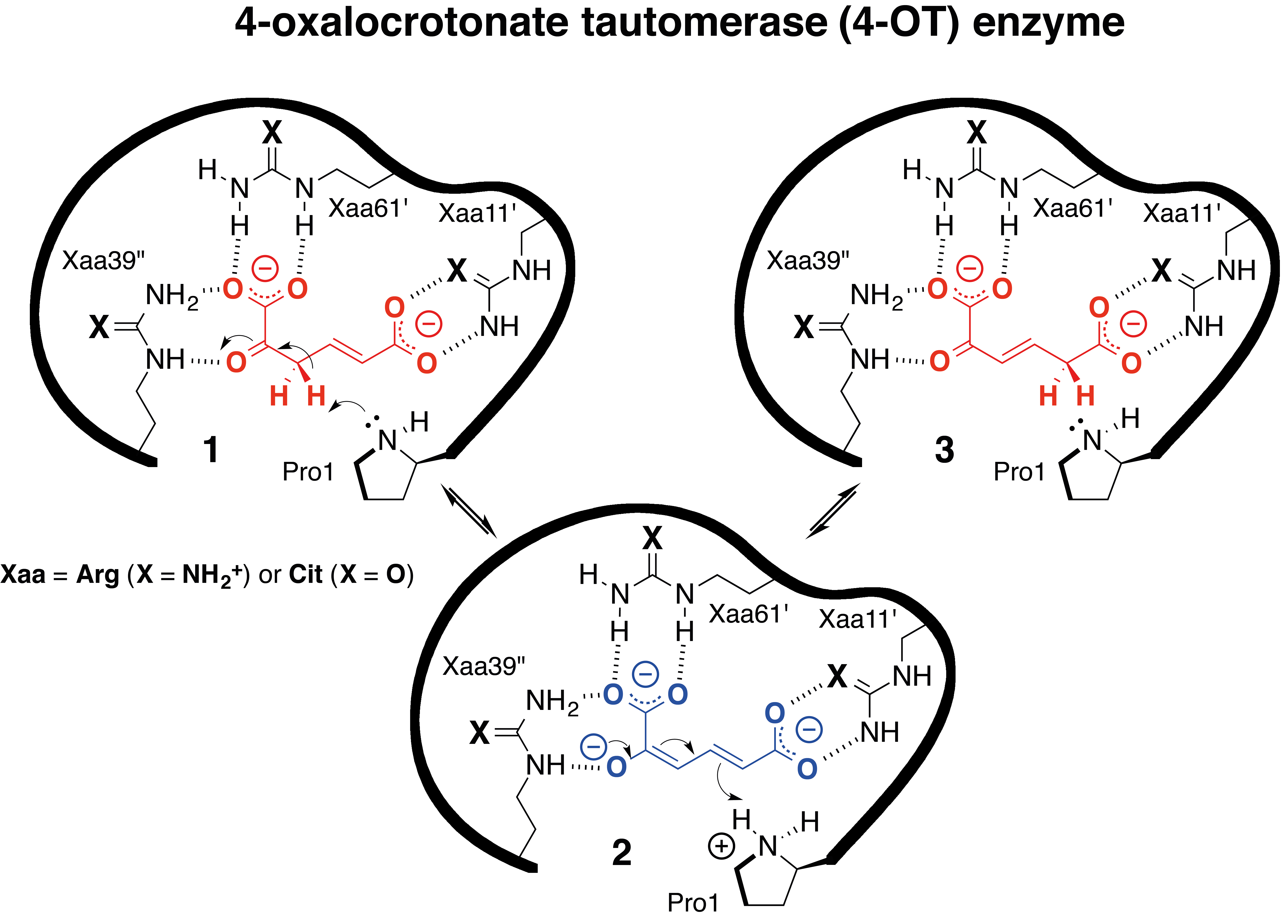
Metanis, N.; Keinan, E. and Dawson, P.E. J. Am. Chem. Soc. 2005, 127, 5862-5868. DOI: 10.1021/ja050110b
• Electrostatic interactions dominate the catalytic contribution of Arg39 in 4-Oxalocrotonate Tautomerase
Metanis, N.; Brik, A.; Dawson, P.E. and Keinan, E. J. Am. Chem. Soc. 2004, 126, 12726-12727. DOI: 10.1021/ja0463841
|
|