Ori Gidron Research Group
Organic Electronic Materials



57. Controlling the Helicity and Handedness of Polyaromatics with Isobenzofuranophane
Agrawal, A. R.; Shioukhi, I.; Deree, Y.; Bogoslavsky, B.; Shalev, O.; Hoffman, R.; Gidron, O.

56. Enhancing the Solid-State Emission of Carbonyl-Containing Compounds by Means of Introducing a Bifuran Core
Yakir, H. R.; Bogoslavsky, B.; Gidron, O.

55. Unravelling Quantum Dot–Molecule Interactions for π-Conjugated Ligands: Insights into Binding and Anchoring Group Effects
Deree, Y.; Levi, A.; Li, X.; Gidron, O.; Banin, U.

54. Magneto-chiral dichroism in a chiral twistacene ytterbium (III) one-dimensional assembly of single-molecule magnets
Shioukhi, I.; Adi, L. C.; Dorcet, V.; Cador, O.; Rikken, G. L. J. A.; Le Guennic, B.; Gidron, O.; et al.

53. The photochemistry and photophysics of benzoyl-carbazole
Deree, Y.; Bogoslavsky, B.; Schapiro, I.; Gidron, O.

52. Exploring color space: an investigation of heteroaryl-substituted benzobis[1,2-d:4,5-d′]oxazoles and their application in organic light-emitting diodes
Wheeler, D. L.; Tannir, S.; Yakir, H. R.; Dishi, O.; Gidron, O.; Jeffries-EL, M.
51. Helitwistacenes: Combining Lateral and Longitudinal Helicity Results in Solvent-Induced Inversion of Circularly Polarized Light
Shioukhi, I.; Batchu, H.; Schwartz, G.; Minion, L.; Deree, Y.; Bogoslavsky, B.; Shimon, L.; Wade, J.; Hoffman, R.; Fuchter, M. J.; Markovich, G.; Gidron, O.*
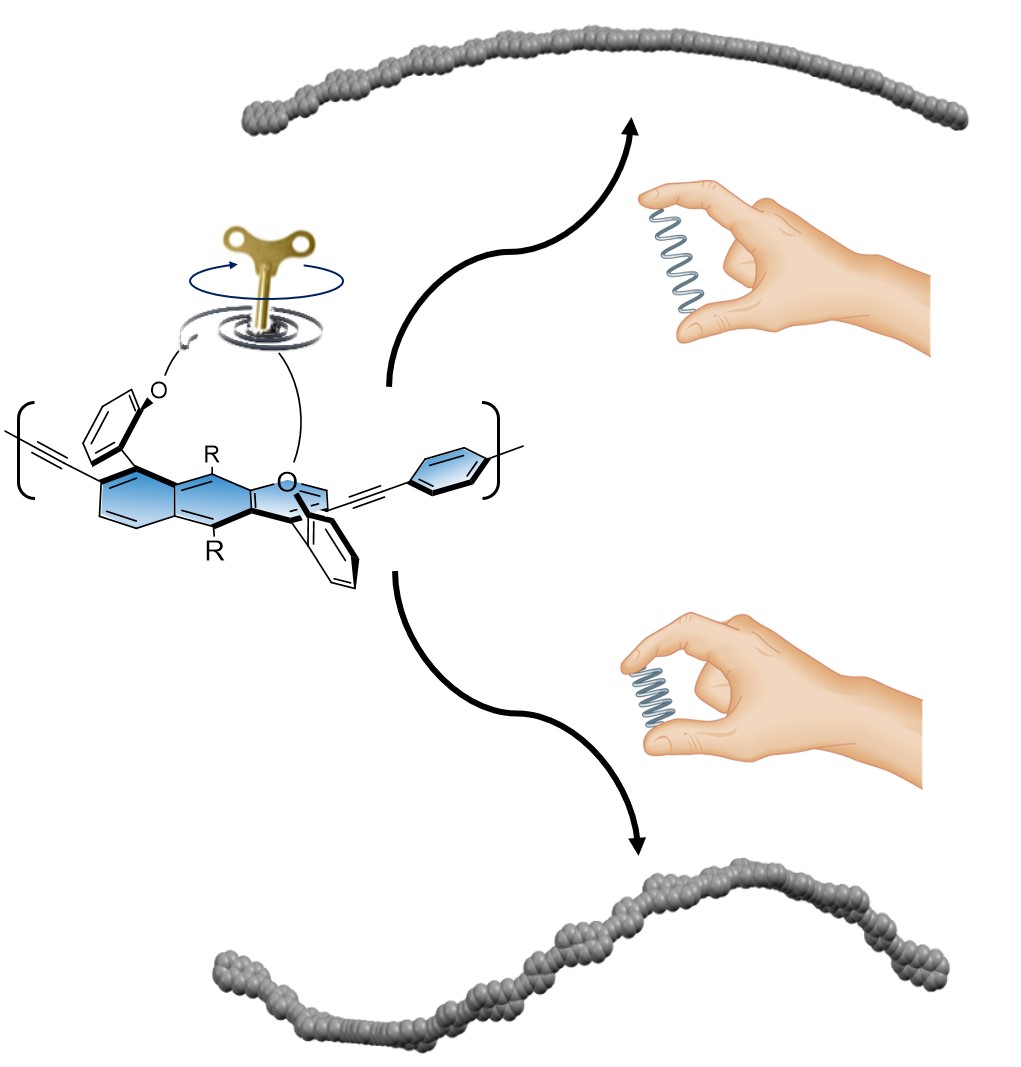
50. Controlling helicene's pitch by molecular tethering
Agrawal, A. R.; Shioukhi, I.; Deree, Y.; Bogoslavsky, B.; Gidron, O.*
49. π-Conjugated oligofuran macrocycles
Dishi, O.; Rahav, Y.; Gidron, O.*
2023
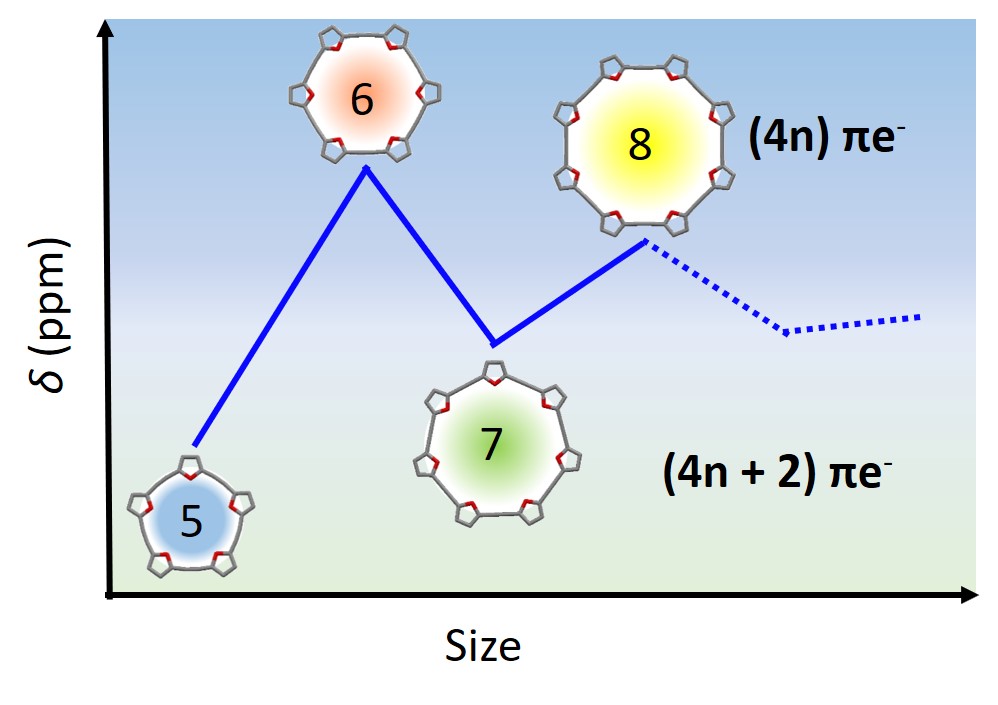
48. Alternating behavior in furan-acetylene macrocycles reveals the size-dependency of Hückel’s rule in neutral molecules.
Rahav, Y.; Rajagopal, S. K.; Dishi, O.; Bogoslavsky, B.; Gidron, O.*

47. Spin polarization through axially chiral linkers: Length dependence and correlation with the dissymmetry factor
Amsallem, D.; Kumar, A.; Naaman, R.*; Gidron, O.*

46. Optical Activity and Spin Polarization - The Surface Effect
Metzger, S. T.; Batchu, H.; Kumar, A.; Fedotov, D.; Goren, N.;Bhowmick, D. K.; Shioukhi, I.; Yochelis, S.; Shapiro, I.; Naaman, R.*; Gidron, O.*; Paltiel, Y.*

45. The Effect of Axial and Helical Chirality on Circularly Polarized Luminescence: Lessons Learned from Tethered Twistacenes.
Bedi, A.*; Schwatz, G.; Manor Armon, A.; Hananel, U.; Shioukhi, I.; Markovich, G.; Gidron, O.*
2022
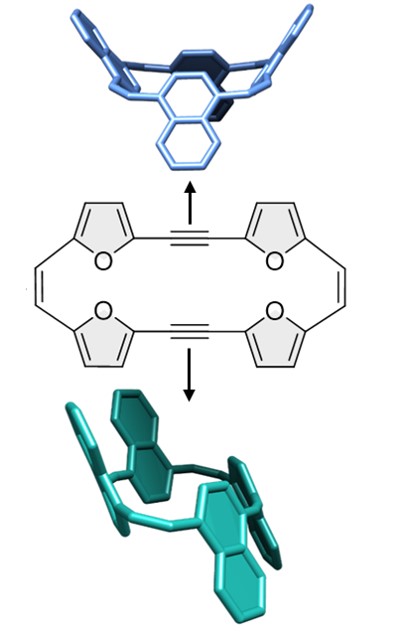
44. Transformation of π-Conjugated Macrocycles: From Furanophanes to Naphthalenophanes
Rajagopal, S. K.; Dishi, O.; Bogoslavsky, B.; Gidron, O.*

43. A Macrocyclic Furan with Accessible Oxidation States: Switching Between Aromatic and Antiaromatic Global Ring Currents
Dishi, O.; Rahav, Y.; Carmieli, R.; Gidron, O.*
Chem. Eur. J. 2022 28, e202202082.
42. Controlling the Helicity of π-Conjugated Oligomers by Tuning the Aromatic Backbone Twist
Bedi, A.; Armon, A.; Diskin-Posner, Y.; Bogoslavsky, B.; Gidron, O.*

41. The Impact of Twisting on the Intersystem Crossing in Acenes: An Experimental and Computational Study
Malakar, P.; Borin, V.; Bedi, A.; Schapiro, I.*; Gidron, O.*; Ruhman, S.*
2021

40. Ring Size Determines the Conformation, Global Aromaticity and Photophysical Properties of Macrocyclic Oligofurans
Dishi, O.; Malakar, P.; Shimon, L. J. W.; Ruhman, S.; Gidron, O.*

39. Raman and ROA Analysis of Twisted Anthracenes: Connecting Vibrational and Electronic/Photonic Structure
Palmono, L.; Gamez, F. G.; Bedi, A.; Gidron, O.; Casado, J.; Ramirez, F. J.
Phys. Chem. Chem. Phys. 2021 23, 13996.
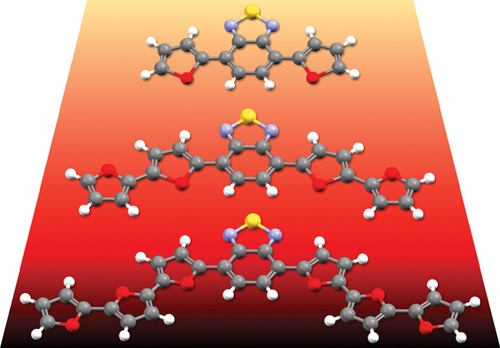
38. Oligofuran–Benzothiadiazole Co-oligomers: Synthesis, Optoelectronic Properties and Reactivity
Ben Abba Amiel, D.; Choongik, K.; Gidron, O.

37. Bending versus Twisting Acenes–A Computational Study
Armon, A.; Bedi, A.; Borin, V.; Schapiro*, I.;Gidron, O.*
2020
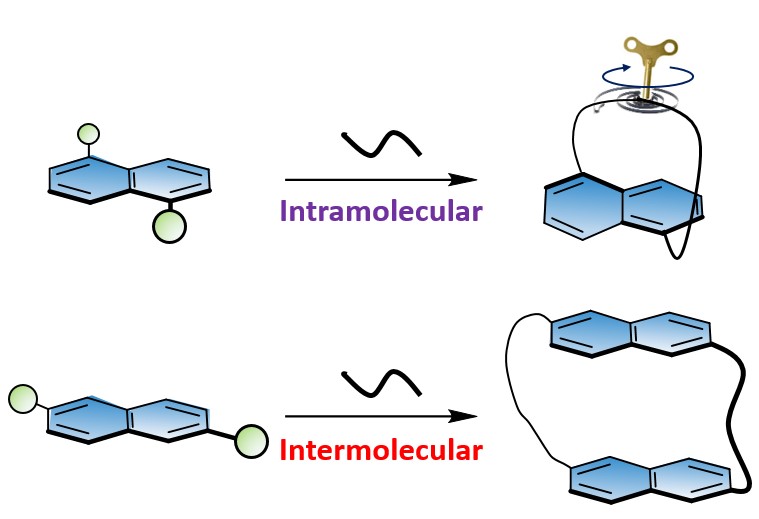
36. Easier to Twist than Bend: The Scope of the Bridge Formation Approach to Naphthalenophane Synthesis
Bedi. A.*; Gidron, O.*

35. Effect of Twisting on the Capture and Release of Singlet Oxygen by Tethered Twisted Acenes
Bedi, A.; Manor, A. M.; Gidron, O.*

34. Relation between Morphology and Chiroptical Properties in Chiral Conducting Polymer Films: A Case Study in Chiral PEDOT
Amsallem, D.; Bedi, A.; Tassinari, F.; Gidron, O.

33. Spin-Dependent Enantioselective Electropolymerization
Tassinari, F.*; Amsallem, D.; Bloom, B. P.; Lu,Y.; Bedi, A.; Waldeck, D. H.; Gidron, O.; Naaman R.
2019

32. A Macrocyclic Oligofuran: Synthesis, Solid State Structure and Electronic Properties
Mulay, S.; Dishi, O.; Fang, Y.; Niazi, M. R.; Shimon, L. J. W.; Perephichka, D. F.*; Gidron, O.*
31. Artificial Photosynthesis with Electron Acceptor/Photosensitizer-Aptamer Conjugates
Luo, G.; Biniuri, Y.; Chen, W.-H.; Neumann, E.; Fadeev, M.; Marjault, H.-B.; Bedi, A.; Gidron, O.; Nechushtai, R.; Stone, D.; Happe, T.;Willner, I.*
30. Young Career Focus: Dr. Ori Gidron (The Hebrew University of Jerusalem)
Gidron, O.*

29. Photoexcited Triplet States of Twisted Acenes Investigated by Electron Paramagnetic Resonance
Tait, C. E.; Bedi, A.; Gidron, O.; Behrends, J.*
28. The Consequences of Twisting Nanocarbons: Lessons from Tethered Twisted Acenes
Bedi, A.; Gidron, O.*
Acc. Chem. Res. 2019 52, 2482.

27. Radical Cations of Twisted Acenes: Chiroptical Properties and Spin Delocalization
Bedi, A.; Carmieli, R.; Gidron, O.*
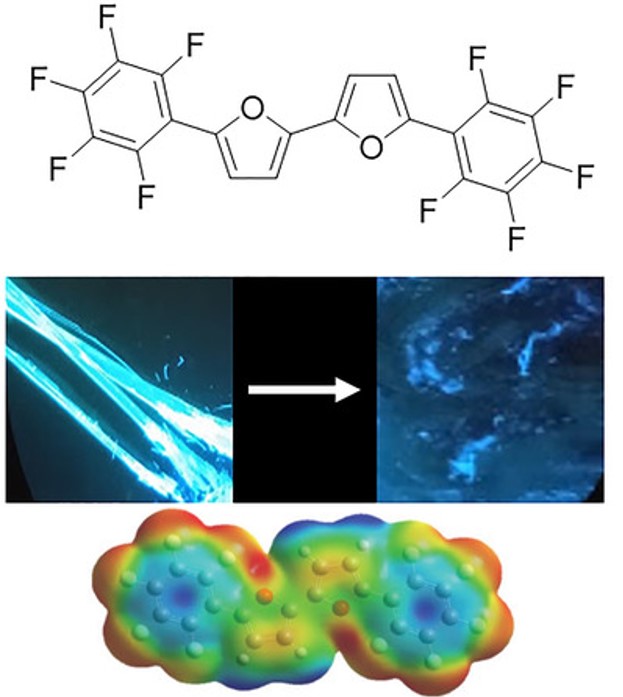
26. Perfluorophenyl-Bifuran: A Stable and Fluorescent Material Exhibiting Mechanofluorochromic Behavior
Yakir, H.; L. J. W. Shimon; Gidron, O.*
Helv. Chim. Act. 2019 102, e1900027.

25. Chiroptical Properties of Twisted Acenes: Experimental and Computational Study
Bedi, A.; Gidron, O.*
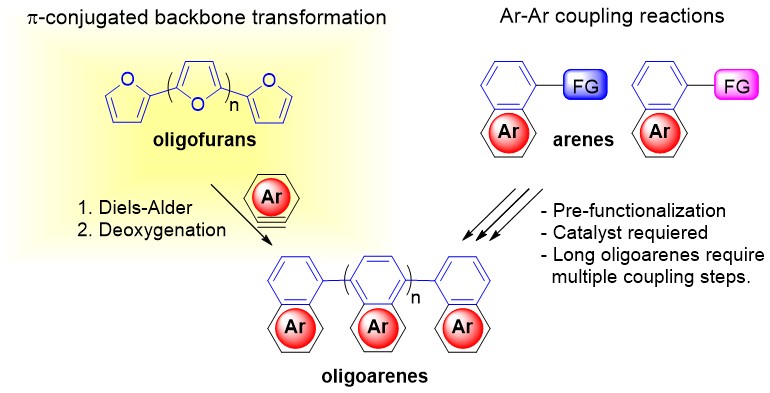
24. Multiple Diels-Alder Transformations in Linear π-Conjugated Systems
Bedi, A.; Gidron, O.*
2018

23. Bifuran-imide: A Stable Furan Building Unit for Organic Electronics
Mulay, S. V.; Bogoslavsky, B.; Galanti, I.; Galun, E.; Gidron, O.*

22. Helically-Locked Tethered Twistacenes
Bedi, A.; Shimon, L. J. W.; Gidron, O.*

21. Macrocyclic Oligofurans: A Computational Study
Dishi, O.; Gidron, O.*
20. Solution-processable dithieno[3,2-b:2′,3′-d]thiophene derivatives for organic thin-film transistors and complementary-like inverters
Ho, D.; Jeon, M.; Kim H.; GidronC, O.; Kim, C.*; Seo, S.*
19. A Stretchable Alternating Current Electroluminescent Fiber
Hu, D.; Xu X.; Miao, J.; Gidron, O.; Hong, M.*
2017
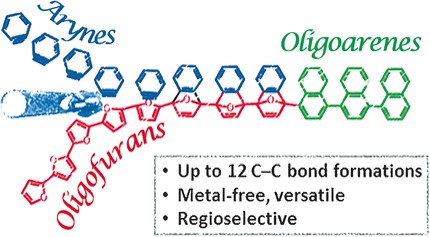
18. Regioselective Transformation of Long π-Conjugated Backbones: From Oligofurans to Oligoarenes
Phatangare, S.; Shimon, L. J. W.; Gidron, O.*
2016

17. Enantiopure Alleno-Acetylenic Helicages Containing Multiple Binding Sites
Gidron, O.; Jirasek, M.; Worle, M.; Diederich, F.*
Before joining the Hebrew University of Jerusalem

16. Homochiral [2]Catenane and Bis[2]catenane from Alleno-Acetylenic Helicates - A Highly Selective Narcissistic Self-Sorting Process
Gidron, O.; Jirasek, M.; Trapp, N.; Ebert, M-O.; Zhang, X.; Diederich, F.*
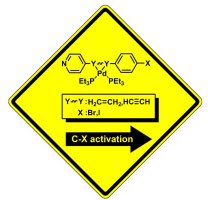
15. Mechanistic Aspects of Aryl-Halide Oxidation Addition, Coordination Chemistry and Ring-Walking by Palladium.
Zenkina, O.V.; Gidron, O.; Shimon, L. J. W.; Iron, M. A.; van der Boom, M. E.*

14. Conducting Polyfurans by Electropolymerization of Oligofurans
Sheberla, D.*; Patra, S.; Wijsboom, Y. H.; Sharma, S.; Sheynin, Y.; Hayoun Barak, A.; Elrazek haj Yahia, A.; Gidron O.; Bendikov M.

13. Oligofurans and Related Compounds
Gidron, O.*
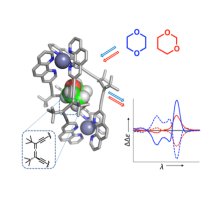
12. Chiroptical Detection of Non-Chromophoric, Achiral Guests by Enantiopure Alleno-Acetylenic Helicages
Gidron, O.; Trapp, N.; Ebert, M-O.; Diederich, F.*
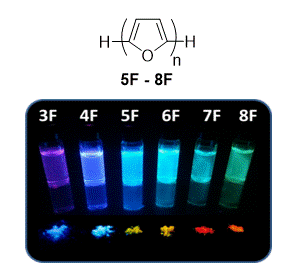
11. Oligofurans – An Emerging Class of Conjugated Oligomers for Organic Electronics
Gidron, O.*; Bendikov, M.

10. Outstanding Chiroptical Properties: A Signature of Enantiomerically Pure Alleno–Acetylenic Macrocycles and Monodisperse Acyclic Oligomers.
Donckle, E.; Gidron, O.; Trapp, N.; Ebert, M-O.; Diederich, F.*
Chem. Eur. J. 2014, 20, 9558-9566.
Inside Cover.

9. Study of Bifuran Linker vs. Bithiophene Linker for Rational Design of π-Conjugated Systems. What Have We Learned?
Gidron, O.; Varsano, N.; Shimon, L. J. W.; Leitus, G.; Bendikov, M.*
Chem. Commun. 2013, 49, 6256-6258.
Front Cover Page.

8. High Charge Delocalization and Conjugation in Oligofuran Molecular Wires
Gidron, O.; Diskin-Posner, Y.; Bendikov, M.*
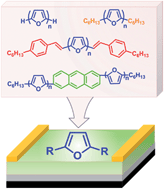
7. Oligofuran-Containing Molecules for Organic Electronics
Gidron, O.; Dadvand, A.; Sun, E. W.-H; Chung, I.; Shimon, L. W. J.; Bendikov, M.; Perepichka, D. F.*
6. Coordination-Based Molecular Assemblies of Oligofurans and Oligothiophenes
Hayoun Barak, A.; de Ruiter, G.; Lahav, M.; Sharma, S.; Gidron, O.; Evmenenko, G.; Dutta, P.; Bendikov M.; van der Boom M. E.*

5. α-Oligofurans Show a Sizeable Degree of π-Conjugation as Probed by Raman Spectroscopy.
Ferrón, C. C.; Delgado, C. R. M.; Gidron, O.; Sharma, S.; Sheberla, D.; Sheynin, Y.; Bendikov, M.*; Navarrete, J. T. L.; Hernández, V.*

4. Reactivity of Long Conjugated Systems: Selectivity of Diels–Alder Cycloaddition in Oligofurans
>Gidron, O.; Shimon, L. J. W.; Leitus, G.; Bendikov, M.*

3. Towards "Green" Electronic Materials. a-Oligofurans as Semiconductors.
Gidron, O.; Dadvand, A.; Sheyning, Y.; Bendikov, M.*; Perepichka, D. F.*
Chem. Commun. 2011, 46, 1976-1978.
Front Cover
2. α-Oligofurans
Gidron, O.; Diskin-Posner, Y.; Bendikov, M.*

1. Structure of Rubrene - Planar or Twisted? Study of Substituted Rubrenes.
Paraskar, A. S.; Reddy, A.R.; Patra, A.; Wijsboom, Y. H.; Gidron, O.; Leitus, L.; Bendikov M.*
2. Soluble Conjugated Oligomers
1. Oligo- and Polyfurans, Preparation and Uses thereof